Harmed by Sunscreen? What Parents Need to Know
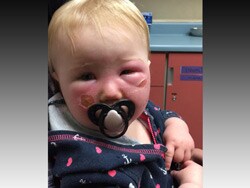
It's been more than 3 weeks, and 14-month-old Kyla Fudge is still recovering from a painful, blistering rash that spread over her cheeks and nose, right where her mom had spread a palmful of Banana Boat Kids Free spray sunscreen.
Her story has incensed parents across the internet. Her mom understands why.
"Nobody, adult or child, deserves to go through that. That was insane. That was the hardest couple of weeks of our lives," says Kyla's mom, Rebecca Cannon, 32, of Newfoundland, Canada.
The family had gone to visit Kyla's aunt and cousins. The older kids wanted to play outside. The day was overcast and slightly chilly, but the sun was peeking in and out from behind the clouds, so Cannon dressed her daughter in a coat and hat and dabbed some of the Banana Boat spray on her nose and cheeks, where her face was exposed.
They'd never used the product before, but Rebecca read the label. It said it was safe for babies over the age of 6 months.
Source: courtesy of Rebecca Cannon
"Her face gradually throughout the day got pinker. I thought it was just irritated from a different sunscreen, so I took her home and gave her a bath," Cannon says. "When she woke up the next day she was really swollen and blistering, so I took her to the ER."
There, doctors admitted that because Kyla's face was so swollen, it was hard to make a definite diagnosis. Their best guess, after consulting with a pediatric dermatologist, was a caustic burn, perhaps caused by something in the sunscreen.
Health Canada, the Canadian counterpart to the U.S. FDA, is investigating.
Renelle Briand, a spokesperson for the agency, says that in addition to Kyla's case, they are aware of three other Canadian children who had reactions to Banana Boat sunscreens in May. That's a tiny fraction of the overall number of people who use this product, but the agency is being cautious, she says, since it's always possible that the manufacturer could have had a bad batch. She says it's too early in the process to even guess at what may have caused the kids' severe reactions.
Cannon posted about the family's ordeal on her Facebook page. One of her friends asked to share Kyla's pictures with her mommy group, and from there, the warning went viral -- generating as many questions from worried parents as answers.
Did Cannon use the product correctly? Cannon says she did, first spraying it into her own hand and then spreading it across the exposed parts of her daughter's face.
Did she simply let Kyla stay out in the sun for too long? She doesn't think so. "Her hands were out in the open, and they didn't burn," Cannon says.
Cannon reported Kyla's reaction to Edgewell, the company that owns Banana Boat. They sent her a check for $10 to reimburse her for the cost of the product.
In a statement to WebMD, the company says their products undergo rigorous testing to ensure safety and quality before they're put on the market. They said all their products have a neutral pH, which means they can't cause chemical burns.
So what happened here?
Alok Vij, MD, a dermatologist at the Cleveland Clinic, thinks Kyla's reaction was something called a photoallergy. That's when sunlight combines with a chemical to bring on an allergic reaction. "It's not very common, but sunscreens are a common cause of that reaction," he says.
"The thing that makes it harder to diagnose is that there are a lot of potential allergens in the chemical sunscreens. Fragrances, preservatives -- a lot of ingredients that sunscreen companies use to make them spread more easily, absorb more quickly, essentially make them more elegant to put on the skin," Vij says.
The company acknowledges that photoallergic reactions can be a possibility with its sunscreens.
"For some, a sensitivity to a product ingredient can be triggered or exacerbated by the sun and result in a photoallergic skin rash or sunburn. In more severe cases, blistering may also develop. A dermatologist can also advise on appropriate treatment," it says in a written statement.
There are several different ingredients in the formula that Cannon used that are known to trigger a reaction in sensitive people. The chemicals avobenzone, octinoxate, and octocrylene have all been linked to cases of photo allergy.
"Banana Boat uses the octocrylene as a base to prevent the sunscreening agent avobenzone from breaking down, says Darrell Rigel, MD, a dermatologist at NYU's Langone Medical Center. He consults with companies on their sunscreen formulations, but he has never worked for Banana Boat.
He says Banana Boat uses octocrylene as a stabilizer. "Different companies have different ways of stabilizing avobenzone. Neutrogena has Helioplex, Aveeno has Active Photo Barrier Complex," he says.
Reactions to octocrylene have been documented in children but also in adults, especially if they are also allergic to the pain reliever ketoprofen.
Still, Rigel wants people to know that reactions to sunscreen ingredients are thought to be rare. "It's only about 1% of the population that gets it," he says.
The FDA agrees. We asked the agency if it is also investigating these reports of severe skin reactions in kids who've recently used Banana Boat.
In an emailed statement, the FDA says it is aware of cases of serious allergic reactions caused by sunscreens. That's why most over-the-counter products advise testing a patch of skin before applying the product over a wider area.
"Although consumers occasionally experience a skin reaction, the potential overall benefits of regular sunscreen use outweigh the potential risks of these reactions. Using sunscreens with other sun protection measures, as directed, can help reduce the risk of skin cancer and premature skin aging caused by the sun," they write.
The agency says people who've had a bad reaction to a sunscreen should stop using it and get medical advice.
As for other measures parents can take to cut the risk of photo allergy in kids, experts say the most important thing to do is to think of sunscreen as your last line of defense against UV rays.
Vij advises parents to:
Keep kids out of the sun during the brightest parts of the day, between 11 a.m. and 4 p.m.
Use sun-protective clothing. "I recommend it because you're not worried about where you've spread sunscreen on a little squirrelly toddler," he says.
Use mineral-based sunscreens, like those with zinc or titanium. Vij says these are harder to spread on the skin, but they're less likely to trigger reactions.
Avoid spray sunscreens, since it's very hard to see where you've put them or to know how much you've applied.
Benjamin Hidalgo-Matlock
Skin Care Physicians of Costa Rica
4000-1054
2208-8206
Please excuse the shortness of this message, as it has been sent from a mobile device.




 Article Info
Article Info