Síndrome de Neterton
NETHERTON SYNDROME: FROM APEX TO NETHER

By Warren R. Heymann, MD
March 31, 2021
Vol. 3, No. 13
NS (OMIM 256500) is a rare autosomal recessive disorder, due to germline mutation of SPINK5, affecting one in 100,000 to 200,000 live births. Congenital ichthyosiform erythroderma (CIE) and/or ichthyosis linearis circumflexa (ILC), hair shaft abnormalities (notably trichorrhexis invaginata, TI) and an atopic diathesis (elevated serum IgE) characterize the syndrome. NS is often misdiagnosed as atopic dermatitis due to the presence of eczematous skin lesions and other allergies. Neonatal cases may be complicated by hypernatremic dehydration and failure to thrive. (1)
NS is caused by variants in the SPINK5 gene (serine protease inhibitor of kazal type 5) encoding LEKTI (lymphoepithelial kazal type‐related inhibitor), which is expressed in the stratified epithelium of the skin, mucosa, and Hassal corpuscles of the thymus. LEKTI deficiency causes a loss of inhibition of serine proteinases such as plasmin, trypsin, elastase, and others This results in unopposed activity of kallikrein‐related peptidase 5 (KLK5), which activates KLK7, KLK14, and elastase 2 (ELA2). Subsequent increased degradation of corneodesmosomal cadherins through increased degradation of desmoglein 1, increased desmosome cleavage, and reduced filaggrin proteolytic processing results. This manifests as detachment of the stratum corneum, thereby contributing to a defective skin barrier, enabling microbe and allergen penetration. (2)
Perhaps the phenotype of CIE can be explained by the finding that patients with NS display increased activity of both transglutaminase 1 (TG1) and serine proteases. Wiegmann et al demonstrated that specific LEKTI domains are crosslinked into the epidermis by TG1. (3)
Williams et al have observed that sequencing of the skin microbiome demonstrates that lesional skin of NS subjects is dominated by Staphylococcus aureus and Staphylococcus epidermidis. In mice, it has been demonstrated that these microbes promote skin inflammation in the setting of LEKTI-1 deficiency due to excess proteolytic activity promoted by S. aureus phenol-soluble modulin α as well as increased bacterial proteases staphopain A and B from S. aureus or EcpA from S. epidermidis. The authors believe these findings demonstrate the crucial need for maintaining homeostasis of host and microbial proteases for disease prevention. (4) Although an atopic diathesis is displayed, there does not appear to be any severe associated systemic immunodeficiency in patients with NS; however, there is conflicting data regarding immunological aberrations. Some of these have been attributed to the observation that IVIG has demonstrated clinical benefit in NS. (2)
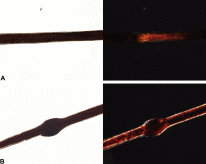
Affected hair in NS appears short, dry, dull, and brittle. Trichorrhexis invaginata (TI) or "bamboo hair" is the invagination of affected hairs caused by softness of the cortex in the keratogenous zone. TI may not be obvious in infants with NS, making the diagnosis of NS difficult. Dermatoscopy and reflectance confocal microscopy may be the best way to observe TI. (5) The absence of TI does not exclude a diagnosis of NS. (1) TI often improves with age and may resolve completely. Utsumi et al described a band-like pattern in hairs with polarized light in patients with NS, which were observed more often than TI. A limitation of the study was that the procedure was not compared to other disorders of keratinization — further research will determine if this has the potential to be used as a diagnostic marker for NS. (6) Therapeutic options for NS are of relatively limited value. Topical steroids, topical calcineurin inhibitors, topical retinoids, phototherapy (narrow band UVB, PUVA), acitretin, IVIG, and TNF inhibitors have all been variably successful. (1) Volc et al reported an excellent response to ustekinumab in a 15-year-old girl, speculating that IL-17 and IL-23 inhibitors may also be of value. (7) The most intriguing therapeutic advance for NS is on the horizon. KLK5 inhibition has been proposed as a potential therapeutic treatment for NS. White et al described how fragmentation of known trypsin-like serine protease (TLSP) inhibitors resulted in the identification of a series of phenolic amidine-based KLK5 inhibitors. (8) The prospect of a precise molecular therapy for NS is scintillating.
Point to Remember: Netherton syndrome may be easily misdiagnosed as atopic dermatitis. The diagnosis may be simplified by dermoscopy, finding either trichorrhexis invaginata or hair banding. The precise molecular treatment of NS utilizing kallikrein 5 inhibitors is currently under investigation.
Our expert's viewpoint
Albert C. Yan, MD
Professor of Pediatrics and Dermatology
Perelman School of Medicine at the University of Pennsylvania
Our evolving understanding of Netherton syndrome exemplifies the current realization that identifying a causative gene mutation such as with SPINK5 is just one of the first steps in finding potential treatment options. It is now just as important to understand the cascade of effects that a single gene mutation has downstream which will help demonstrate potential therapeutic targets. While the importance of knowing that SPINK5 underlies Netherton syndrome cannot be understated, recently employed treatment options have exploited some of these other downstream effects. The editorial above has already highlighted new work indicating potential benefits of targeting KLK5 inhibitors. In the paper by Williams et al., Staphylococcus aureus plays a significant role in influencing the Netherton syndrome phenotype through mechanisms that increase protease activity and skin barrier disruption. Infectious organisms like Staphylococcus which may proliferate in disrupted skin are known to trigger a Th17 response and treatment of these downstream effects using medications that target IL17 and IL23 have shown some clinical benefit in patients with Netherton syndrome. Teasing out additional pathways affected by mutations in SPINK5 should help not only elucidate our understanding of the disease but also provide additional therapeutic targets for further investigation. Dr. Yan had disclosed financial relationships with the following to the AAD at the time of publication: Aclaris Therapeutics, Inc., BabyDoctor.com, Dermavant Sciences, Johnson & Johnson Consumer Products Company, Ortho Dermatologics, Pfizer Inc., Procter & Gamble Company, Regeneron Pharmaceuticals, Inc., Verrica Pharmaceuticals Inc. Full disclosure information is available at coi.aad.org.
Saleem HMK, Shahid MF, Shahbaz A, Sohail A, et al. Netherton syndrome: A case report and review of literature. Cureus 2018;10:e3070. doi: 10.7759/cureus.3070.
Stuvel K, Heeringa JJ, Dalm VASJ, Meijers RW, et al. Comel-Netherton syndrome: A local skin barrier defect in the absence of an underlying immunodeficiency. Allergy 2020 Jan 24. doi: 10.1111/all.14197. [Epub ahead of print]
Wiegmann H, Valentin F, Tarinski E, Liebau E, et al. LEKTI domains D6, D7 and D8+9 serve as substrates for transglutaminase 1: Implications for targeted therapy of Netherton syndrome. Br J Dermatol 2019; 181: 999-1008.
Williams MR, Cau L, Kaul D, Wang Y, et al. Interplay of Staphylococcal and host proteases promotes skin barrier disruption in Netherton syndrome. Cell Rep 2020 Mar 3;30(9):2923-2933.e7. doi: 10.1016/j.celrep.2020.02.021.
Chen L, Yang Y, Tian X, Li D, et al. Dermatoscopy of the hair compared to three alternatives for the diagnosis of pediatric Netherton syndrome. J Dermatol 2020 Mar 15. doi: 10.1111/1346-8138.15307. [Epub ahead of print].
Utsumi D, Yasuda M, Amano H, Suga Y, et al. Hair abnormality in Netherton syndrome observed under polarized light microscopy. J Am Acad Dermatol 2020 Feb 3. pii: S0190-9622(19)32571-X. doi: 10.1016/j.jaad.2019.08.024. [Epub ahead of print].
Volc S, Maier I, Gritsch A, Aichelburg MC, et al. Successful treatment of Netherton syndrome with ustekinumab in a 15-year-old girl. Br J Dermatol 2020 Jan 24. doi: 10.1111/bjd.18892. [Epub ahead of print].
White GV, Edgar EV, Holmes DS, Lewell XQ, et al. Kallikrein 5 inhibitors identified through structure based drug design in search for a treatment for Netherton syndrome. Bioorg Med Chem Lett 2019 Mar 15;29(6):821-825.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
Skin Care Physicians of Costa Rica
Clinica Victoria en San Pedro: 4000-1054
Momentum Escazu: 2101-9574
Please excuse the shortness of this message, as it has been sent from
a mobile device.
posted by dermatica at
March 31, 2021
|
0 Comments
![]()
![]()




This study builds on prior work by Day et al1,2 and Sober et al3 that found various risk factors (including the anatomical location of melanomas) affected mortality and outcomes, and it seeks to determine if there are different etiologies for melanomas arising on different anatomic locations. The "dual pathway hypothesis" suggests that melanoma of the trunk (and less sun–exposed areas) is driven by materially different, more intrinsic factors than melanoma of the head and neck (more sun–exposed areas). To do so, the authors of this study investigated the variation between different risk factors (hair/eye color, number of nevi, skin tone, UV exposure, polygenic risk scores) and melanomas found on different anatomical sites (trunk, head and neck, upper limbs, and lower limbs).
The study collected data and DNA samples from 2617 patients, primarily of European descent, with a first-time diagnosis of invasive melanoma and 975 age-, sex-, and geographically matched controls using data obtained from an Australian and UK case–control cohort. Overall, the authors found that an increasing number of nevi corresponded with truncal melanoma and that, after controlling for additional phenotypic differences, weekday UV exposure (a proxy for weekday sun exposure) was only associated with head and neck melanomas. The authors propose that these data support the notion of dual pathways for melanomas of the trunk versus head and neck with potential convergence of these pathways for melanomas of the limbs.
Interestingly, UV exposure was not independently associated with increased risk of melanoma at other anatomic sites. The authors suggest that this may be due to a potential modifying association with skin phenotype. There was also not a significant variation in the association between polygenic risk factors and melanoma across anatomic sites, which, the authors note, may be due to their polygenic risk score only accounting for a limited variety of phenotypes. Additional limitations include the retrospective nature of the study, relatively smaller control cohort, as well as younger age of the Australian cohort.
These findings have the potential to improve patient care and counseling by improving dermatologists' understanding of melanoma pathogenesis. Future studies should endeavor to study additional anatomical sites (palms, soles, nails) across more diverse racial and ethnic populations to improve generalizability of findings and how sun-protective measures and clothing differentially impact melanoma pathogenesis.
References