Based on a presentation by Bruce Strober, MD, PhD—Clinical Professor of Dermatology at Yale University School of Medicine and is with Central Connecticut Dermatology
Apremilast. Many drugs are versatile, and indications often expand for mature drugs over the course of time. Case in point: the United States (US) Food and Drug Administration (FDA) has recently cleared apremilast, an established oral phosphodiesterase-4 (PDE4) inhibitor, for the treatment of scalp psoriasis. The Study of the Efficacy and Safety of Apremilast in Subjects with Moderate to Severe Plaque Psoriasis of the Scalp (STYLE), a Phase III, double-blind, placebo-controlled study, investigated the use of apremilast 30mg in 303 patients with moderate to severe scalp psoriasis (defined as a Scalp Physician's Global Assessment [S-PGA] score of 3 or greater with psoriasis-involved scalp surface area 20% or greater).1 For inclusion, patients had to have a Psoriasis Area Severity Score (PASI) of 12 or more with at least 10-percent body surface area (BSA) involvement and an S-PGA score of 3 or more (moderate to severe) and an inadequate response or tolerability issues with at least one previous topical treatment for plaque psoriasis of the scalp. The study was designed with a five-week screening period, followed by a 16-week placebo-controlled phase for the primary endpoint, and then an apremilast extension phase of 16 more weeks, followed by four weeks of safety observation.1 Patients in this study had a large scalp surface area affected (58.2%±26.4% and 61.9%±27.2% for control and apremilast groups, respectively), a large BSA for skin psoriasis (21.2%±14.8% and 19.0%±10.8%, respectively) and a Dermatology Life Quality Index (DLQI) score (12.6±7.2 and 12.6±7.0, respectively), demonstrating that this patient population was severely affected by psoriasis. Results showed that at 16 weeks, about 40 percent of apremilast patients had achieved an S-PGA score of 0 or 1 with at least a two-point reduction from baseline, compared to about 14 percent of placebo patients. At 16 weeks, the placebo patients were rotated to apremilast, and the control patients surpassed the active group patients, with the gap widening to Week 32. In terms of achieving at least a four-point improvement from baseline in scalp itch, 46.3 percent of apremilast patients and 18.9 percent of placebo patients reached this endpoint at Week 16, but when the placebo group was switched to apremilast, their results were as good or better at Week 20 and could be maintained at Week 32, when the results converged.1 This differential between apremilast and placebo aligns with results from other studies, as were reported adverse events. The most commonly reported adverse events were diarrhea (31% apremilast, 11% placebo), nausea (22% apremilast, 6% placebo), headache (12% apremilast, 5% placebo), and vomiting (6% apremilast, 2% placebo). Apremilast side effects decreased after 16 weeks (2% diarrhea, 4% nausea, 1% headache, 1% vomiting) while placebo side effects remained the same or increased with the exception of headache which went from 5% to 4%.1
Bimekizumab. Bimekizumab is a new monoclonal antibody that has not yet been approved, but appears promising for the treatment of moderate to severe psoriasis, and is likely to be approved by the end of 2021. Bimekizumab is a selective interleukin (IL) 17A and IL-17F inhibitor. It differentiates itself from the other IL-17A inhibitors, such as secukinumab, ixekizumab, and others, in that also inhibits IL-17F. In the Bimekizumab Efficacy and Safety in Moderate and Severe Plaque Psoriasis (BE READY) study, investigators at 77 sites evaluated bimekizumab 320mg every four weeks versus placebo in 435 patients with moderate to severe plaque psoriasis.2 The study was designed with coprimary endpoints of a PASI 90 and a score of 0 or 1 on the five-point Investigator's Global Assessment (IGA) at 16 weeks. Almost all patients in the bimekizumab group (90.8%) achieved PASI 90 at 16 weeks compared to 1.2 percent of placebo patients with results durable to 52 weeks (86.8% of apremilast patients were PASI 90 at one year). A subset of patients (n=100) who were originally in the bimekizumab group receiving doses every four weeks transitioned at 16 weeks to being dosed every eight weeks and slightly more of these patients (91.0%) maintained PASI 90 at one year. This suggests that if this drug is approved, the dosing regimen might be dosing every four weeks for the initial 16 weeks, then every eight weeks thereafter, which would be an unique dosing regimen for an IL-17 inhibitor. The most frequently reported adverse effect was candidiasis, which occurred in 7.7 percent of bimekizumab and no placebo patients in the first 16 weeks; from weeks 16 to 56, candidiasis occurred in 10.0 to 15.1 percent of bimekizumab patients, respectively.2 No patients in the study had inflammatory bowel disease.
The Bimekizumab Versus Ustekinumab for the Treatment of Moderate to Severe Plaque Psoriasis (BE VIVID) was a placebo-controlled trial with endpoints at 16 and 52 weeks.3 BE VIVID found PASI 90 was achieved by 85.0 percent of bimekizumab patients compared to 49.7 percent of ustekinumab patients at 16 weeks, and those results were 81.6 percent vs. 55.8 percent at 52 weeks (4.8% of placebo patients achieved PASI 90 at 16 weeks). Serious treatment-emergent adverse events (TEAEs) occurred in six percent of bimekizumab patients (n=395) and eight percent of the ustekinumab patients (n=163).3 Oral candidiasis occurred in 18.2 percent of bimekizumab patients up to Week 52, compared to 0.6 percent in the ustekinumab groups. Further, the BE SURE trial compared bimekizumab to adalimumab and found PASI 90 was achieved by 86.2 percent of bimekizumab and 47.2 percent of adalimumab patients at 16 weeks.4 The dose was 320mg bimekizumab every four weeks. At 16 weeks, IGA scores were 0 or 1 for 85.3 percent of bimekizumab and 57.2 percent of adalimumab patients.
It is important to discuss candidiasis among patients taking IL-17 antagonists in general. Among the older IL-17 blockers (secukinumab, ixekizumab, and brodalumab), the rates of candidiasis were typically between two and five percent, with variations depending on the drug, the dose, and the study design.5–8 Candidiasis occurred at higher rates with bimekizumab, ranging from about 10 to 18 percent.2–4 As a rule of thumb, it appears that about five times as many bimekizumab patients are going to have candidiasis than patients taking the older IL-17 inhibitors.
Ixekizumab. Ixekizumab, an IL-17 inhibitor, has recently been approved for pediatric psoriasis. The IXORA-PEDS study was a placebo-controlled trial of patients with psoriasis aged 6 and 17 years.9 Patients were excluded if they had previously taken an IL-17 inhibitor or etanercept. The study permitted the use of concomitant treatments, such as topical steroids, emollients, and bath preparations. All patients had PASI 12 or higher, S-PGA 3 or higher, and BSA 10% or more at the time of screening and randomization. Ixekizumab was administered every four weeks using weight-based dosing. Results in this pediatric population showed that ixekizumab was superior to placebo in PASI and S-PGA responses at 12 weeks. Eighty-nine percent of ixekizumab patients achieved PASI 75 at Week 12 versus 25 percent of placebo patients, with close to 80 percent of ixekizumab patients reaching PASI 90 and 50 percent reaching PASI 100. Of note, before administering this drug in any patient population, prescribers should screen for inflammatory bowel disease.
Secukinumab. At the time of this presentation, secukinumab was not yet approved for pediatric psoriasis, but has since received FDA approval for the treatment of moderate to severe plaque psoriasis in pediatric patients six years and older who are candidates for systemic therapy or phototherapy.10 Dr. Strober discussed the promising clinical trial data from a Phase III, placebo-controlled trial similar to the IXORA-PEDs study.11 In this similar pediatric population, about 70 percent of patients achieved PASI 90 depending on dose compared to 29 percent with etanercept and two percent with placebo. Results showed that at 52 weeks, about half of patients achieved PASI 100. Results varied slightly depending on whether high-dose or low-dose secukinumab was used.11
Drug survival and tailored dosing. Biologic therapy has revolutionized the treatment of psoriasis, but drug survival can emerge as a problem, as patients can become non-adherent or discontinue the medication altogether. Patients might stop taking biologics for any number of reasons, including lack of efficacy, financial burden, or adverse effects.12 Among IL-17 inhibitors, ixekizumab and ustekinumab appear to offer the greatest survivability, with the former superior to the latter.13 As relatively new drugs, the IL-23 blockers have not yet been rigorously analyzed for drug survivability, but will likely be enrolled in similar analyses in the future. There may be ways to improve drug survivability, such as patient education and individualized patient-specific dosing.
IL-17 versus IL-23 inhibitors. There are some important trials comparing IL-17 inhibitors head-to-head against IL-23 inhibitors. In these studies, secukinumab is often selected as the IL-17 blocker, while there is more variation among the IL-23 blockers. These trials are all completed in one year or less. The OASIS-2 trial compared secukinumab 300mg to mirikizumab 250mg every four or eight weeks; the primary endpoint was PASI 90.14 Mirikizumab is an IL-23 inhibitor that has not yet been cleared by the FDA for market release. Here, 73 and 69 percent of secukinumab patients achieved PASI 90 at Week 16 and Week 52, respectively, while 74 and 82 percent of mirikizumab patients achieved PASI 90 at these same time points.
The ECLIPSE study compared secukinumab 300mg to guselkumab 100mg every eight weeks.15 Here, 76 and 70 percent of secukinumab patients achieved PASI 90 at Week 12 and Week 48, respectively, while 69 and 84 percent of guselkumab patients achieved PASI 90 at these same time points.
Finally, the IMMerge study tested secukinumab 300mg against risankizumab 150mg every 12 weeks.16 Here, 66 and 57 percent of secukinumab patients achieved PASI 90 at Week 16 and Week 52, respectively, while 74 and 87 percent of risankizumab patients achieved PASI 90 at these same time points.
A clear theme emerged from these several studies pitting secukinumab, an IL-17 blocker, against a variety of IL-23 blockers. By Week 12 to 16, both inhibitors were comparably effective, but at around one year, the IL-23 inhibitor appeared to be more effective.
Secukinumab dosing for patients with overweight and obesity. Secukinumab was approved in 2015 at a dose of 300mg every four weeks, but many non-responders to secukinumab have overweight or obesity. Dosing modifications are being studied to see how that might affect results with secukinumab in patients with overweight or obesity. The open-label OPTIMISE study investigated a two-week dosing schedule for secukinumab for patients weighing greater than or equal to 90kg.17 The two-week dosing showed greater efficacy in this population in terms of achieving PASI 90 at 16 weeks compared to four-week dosing regimens.17 This might eventually result in labeling changes, and it represents a clinically relevant finding, as obesity is comorbid with psoriatic disease.
Safety of IL-17 and IL-23 inhibitors. Safety data are crucial to consider when making prescribing choices. When malignancy was evaluated in secukinumab-treated psoriasis patients, it was discovered that the rate of cancer was actually lower among secukinumab patients than the general population. In fact, IL-17 blockers do not appear to have any prominent cancer signals.18 Further, when clinical trial data for serious infections and malignancies among patients taking IL-17 inhibitors (ixekizumab, secukinumab, bimekizumab) or IL-23 inhibitors (guselkumab, risankizumab, tildrakizumab) are compared to data from the PSOLAR registry, a long-term registry of patients with psoriasis being treated with systemic therapies that is often used to evaluate long-term safety, IL-17 and IL-23 inhibitors are generally in a low range for these adverse events.19–26 Thus, these drugs do not pose a major risk for malignancy and may even be considered for patients with moderate to severe psoriasis who are at risk for cancer or with a history of cancer.
Nail psoriasis. To some degree, nail psoriasis is addressed by any drug that treats psoriasis, and one agent, adalimumab, is labeled for the treatment of nail psoriasis. Etanercept and tumor necrosis factor (TNF) blockers can also be effective in treating nail psoriasis.27 Recent clinical evidence suggests that IL-17 inhibitors may be among the best agents for nail psoriasis. In a post-hoc subgroup analysis of nail psoriasis in patients with psoriatic arthritis, at 52 weeks, patients who took 80mg ixekizumab twice a week for 12 weeks and then four times a week for the rest of the year were compared to patients who took 40mg adalimumab every two weeks. At 12 weeks, 60 percent of ixekizumab and 49 percent of adalimumab patients had complete resolution of fingernail psoriasis. This pattern was durable over the course of the study, and by 52 weeks, 83 percent of ixekizumab and 72 percent of adalimumab patients had complete resolution of nail psoriasis.28,29
Generalized pustular psoriasis. Although generalized pustular psoriasis (GPP) is not a common condition, it is exciting that a novel agent is undergoing evaluation for treating this challenging condition. Spesolimab (BI 644130) is a promising IL-36 receptor antibody that has not yet been approved. To date, only data from one small (n=7) Phase I study has been reported as a proof-of-concept study for safety and efficacy.30 Patients were included in this study if they had a moderate to severe flare of GPP with greater than or equal to 10-percent BSA with pustules and erythema along with a Generalized Pustular Psoriasis Physician Global Assessment (GPP-PGA) score greater than or equal to 3. Patients were administered a single dose of intravenous (IV) spesolimab 10mg/kg at Day 1 and were then monitored for 20 weeks. All patients had mild to moderate adverse events upon infusion with no serious adverse events reported. By the first week, 5/7 patients achieved a GPP-PGA score of 0 or 1 (clear or almost clear) and 100 percent achieved this goal at four weeks.30 It should be noted that spesolimab was effective in patients with or without the IL-36RN mutation.
The limited prescribing choices for GPP today were the subject of a survey among North American dermatologists. Among the nonbiologic prescribing choices, cyclosporine, isotretinoin, methotrexate, and oral steroids were used most frequently. Although respondents considered them adequate, 72 percent stated they thought these agents were slow at controlling flares.31 The most frequently prescribed biologics were infliximab, ixekizumab, and secukinumab, all of which are known to be safe and effective.31 Until the market arrival of another drug, such as possibly spesolimab, an IL-17 inhibitor should be considered for patients with GPP, as they are effective and produce durable results.
JAK inhibitors. The Janus kinase (JAK) inhibitors are being studied in a variety of immune diseases. Inhibitors work at one of the known JAK pathways (JAK1, JAK2, JAK3) or the related tyrosine kinase (TYK) pathway (TYK2). Drugs that target these pathways interrupt specific JAK Signaling Transducer and Activator Transcription (JAK-STAT). A number of JAK inhibitors are expected to be approved to market soon—most for the treatment of atopic dermatitis but some for psoriasis. An important new agent among them is deucravacitinib, an oral, once-daily TYK2 inhibitor being evaluated for several immune-mediated diseases, including psoriasis. Deucravacitinib is an intriguing new molecule, in that it is selective; it binds to the regulatory domain of TYK2, in such a way that it inhibits TYK2 kinase activity without binding to any other JAK receptors. In that way, deucravacitinib impairs the ability of TYK2 to bind efficiently with JAK1 and JAK2 receptors, thus, indirectly blocking their activity. In a Phase II double-blind study in 267 patients with moderate to severe psoriasis, patients received various doses of oral deucravacitinib (3mg every other day, every day, or multiple times a day) or placebo.32 At 12 weeks, 75 percent of patients in the 12mg/day group achieved a PASI 75 score or better (75% vs. 7% for placebo, p<0.001). The proportions of patients in each group who achieved PASI 75 or higher were: 9 percent in the deucravacitinib 3mg every other day group, 39 percent of the 3mg/day group, 69 percent of the 3 mg/twice daily group, 67 percent for 6 mg/twice daily, and 75 percent of those taking 12 mg/day. Five serious adverse events were observed in four patients (2 in the placebo group, 1 in the 3mg/every other day group, 1 in the 3mg/day group, and 1 in the 3mg/twice a day group). These adverse events included an accidental eye injury, gastroenteritis associated with a rotavirus, dizziness, and an in-situ malignant melanoma (Stage 0). Overall, 51 percent of placebo and 55 to 80 percent of active treatment groups had adverse events with the greatest rate of adverse events occurring in the group who received 6mg/twice a day.32 Deucravacitinib could be an important new drug for psoriasis patients for several reasons. First, it seems to be a highly effective agent with rapid results (over 40% of patients can achieve PASI 90 in 12 weeks32).Second, it is an oral agent that may work with once-a-day dosing, which is generally agreeable to patients and may promote patient adherence. Third, deucravacitinib will not require laboratory testing like other JAK inhibitors, but it is not yet clear how much monitoring is needed for patients.
Another TYK2 inhibitor in the pipeline is the oral agent PF-06826647 for moderate to severe psoriasis. It is an active site binder, not a selective agent like deucravacitinib. In a Phase I, randomized, placebo-controlled trial, patients with plaque psoriasis were treated with PF-06826647 400mg daily, PF-06826647 100mg daily, or placebo for four weeks. The mean improvement in baseline PASI score in the higher dose group was over 80 percent, and 80 percent of these patients achieved PASI 75 at Week 4.33
A better-known JAK inhibitor is tofacitinib, which is approved for psoriatic arthritis, but not psoriasis. Tofacitinib is sometimes used off-label to treat palmoplantar psoriasis, a condition that can be very distressing to patients. According to Dr. Strober's experience in his own clinical practice, the use of tofacitinib 5mg twice a day or tofacitinib extended release 11mg once daily can be effective in treating palmoplantar psoriasis, which can be extremely difficult to treat.
Recently, the International Psoriasis Council led a Delphi group to better help classify psoriasis in patients to improve treatments.34 While objective criteria are often used to classify psoriasis, the expert consensus was that psoriasis patients should first be grouped as to whether they are appropriate candidates for topical therapy or appropriate candidates for systemic therapy. Systemic therapy candidates could be identified by at least one of the following characteristics: BSA greater than 10 percent; disease involving special areas including palms, scalp, soles of feet, genitals, nails; and/or failure of topical therapy. Patients with one or more of these characteristics may benefit from a wealth of systemic agents (such as methotrexate, cyclosporine, apremilast, acitretin, and tofacitinib), phototherapy, and biologics (such as infliximab, etanercept, adalimumab, and others). New biologics are likely to be approved for cutaneous psoriasis soon and biosimilars may also be considered here. These treatments can be used alone or combined with each other, particularly if the patient has not responded well to monotherapeutic regimens. A modern treatment paradigm is summarized in Table 1.

Psoriatic Arthritis Update
Based on a presentation by Arthur Kavanaugh, MD, Professor of Medicine at University of California at San Diego
In about 80 percent of patients, skin psoriasis precedes the development of psoriatic arthritis (PsA), on average about 10 years after the onset of cutaneous symptoms. About 25 percent of patients with cutaneous psoriasis will develop PsA at some point.35,36 In addition to articular disease features, PsA is also associated with enthesitis, dactylitis, inflammatory bowel disease (ulcerative colitis, Crohn's disease), and anterior uveitis/iritis. PsA is also comorbid with major adverse cardiovascular events (MACE) and metabolic syndrome.37,38
The putative etiopathogenesis of PsA has been described.39–41 Recent experience with the COVID-19 pandemic has demonstrated all too clearly how rapidly pathogenic infections can disrupt multiple body systems. A parallel can be drawn to psoriasis, because multiple bodily environments, such as the genome, metabolome, proteome, microbiome, and the epigenome, all become exposed to psoriasis. In particular, the microbiome, in our gut and skin, can lead to PsA signs and symptoms in a variety of domains.41 Much remains to be elucidated, but clinical trials tend to demonstrate that targeted therapies are specific to one or more individual domains.
The resolution of PsA may be viewed as symptomatic improvement in both the skin and joints. When both skin and joint symptoms are relieved, patients experience optimal improvement and quality of life.42
Numerous treatment modalities are available for PsA. Adjunctive agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, and nonpharmacological approaches such as physical therapy are used to address pain; topical products may provide relief for cutaneous symptoms. Disease-modifying antirheumatic drugs (DMARDs), including older agents such as methotrexate, still play an important role in treating PsA. Biologics may be important agents for PsA and biosimilars are increasingly being used. IL-12/23, IL-17, and IL-23 inhibitors are sometimes used, although their effect is greater on cutaneous than rheumatological symptoms. Although apremilast, a PDE4 inhibitor, may not be the most effective treatment against PsA, its robust safety profile makes it a good prescribing choice for certain patients.
A clinical trial randomized 851 PsA patients to three groups: the first group received oral methotrexate 20mg with a subcutaneous placebo weekly; the second group received subcutaneous etanercept 50mg and an oral placebo weekly; the third group received subcutaneous etanercept 50mg and oral methotrexate 20mg weekly. Patients were followed for 24 weeks and evaluated for American College of Rheumatology (ACR) 20-percent improvement (ACR20) and minimal disease activity (MDA).43 Results are summarized in Table 2. Results showed that etanercept monotherapy and combination therapy with etanercept and methotrexate were more effective than methotrexate monotherapy.43 This study lacked a control group so the effect size of methotrexate cannot be stated.

An armamentarium of new agents has been released to market or are in the pipeline for treating various types of inflammatory autoimmune disorders. Not all of the newest agents for inflammatory autoimmune diseases are effective in the treatment of PsA.41 Elucidation of the immunopathology of PsA and cutaneous psoriasis will help better categorize the most effective new agents for various conditions.
Ustekinumab is an IL-12/23 inhibitor with a strong track record in cutaneous psoriasis but it is less well studied for PsA. When compared to TNF-alpha inhibitors (adalimumab, certolizumab, etanercept, infliximab), ustekinumab worked well not just for cutaneous symptoms, but also in peripheral joints and enthesitis.44 Enthesitis is an important and prevalent symptom of PsA and may give rise to painful symptoms in the pelvic region, chest cavity, or other parts of the body. Interestingly, ustekinumab does not appear to be particularly effective for spinal symptoms.
The welcome publication of more head-to-head studies in PsA provides more prescribing guidance. The SPIRIT head-to-head 52-week open-label, assessor-blinded trial compared ixekizumab to adalimumab in 566 patients with PsA. At one year, more ixekizumab than adalimumab patients achieved PASI 100 scores (64% vs. 41%, p<0.001) and the combined endpoint of both ACR50 plus PASI 100 (39% vs. 26%, p<0.001). Results for enthesitis, dactylitis, and ACR50 were similar for both agents.45 In this study, patients were allowed to continue to take methotrexate but it was not required. An intriguing point in this study was that in terms of ACR20/50/70 data at 52 weeks, methotrexate seemed to exert a beneficial combination effect with ixekizumab, but not with adalimumab.45
The secukinumab versus adalimumab for treatment of active PsA (EXCEED) trial was a 52-week, head-to-head comparison that randomized 853 patients with PsA to receive 300mg of subcutaneous secukinumab at baseline, Weeks 1, 2, 3, and 4, and then every four weeks for 48 weeks or subcutaneous adalimumab 40mg every two weeks until Week 50. Results were mixed. More secukinumab than adalimumab patients achieved PASI 90 (65.4% vs. 43.2%, respectively), ACR20 (67.4% vs. 61.5%), and ACR50 (49.0% vs. 44.8%). However, secukinumab was more effective in treating enthesitis, where 60.5 percent of secukinumab patients had resolved symptoms compared to 54.2 percent of adalimumab patients.46
Apremilast is being used with increasing frequency as combination therapy in rheumatology according to the Corrona Registry. Apremilast was determined to be safe and effective in 219 biologic-naïve PsA patients who were randomized to receive either apremilast 30mg twice a day or placebo for 52 weeks in the ACTIVE trial.47 This study found that a subset of PsA patients were taking apremilast as part of combination therapy. Its favorable safety profile again emerged in this study.
Guselkumab is an IL-23p19 antagonist that was approved in 2020 for PsA and was recently evaluated in a double-blind, placebo-controlled, randomized multicenter clinical trial (N=624). PsA patients were randomized to one of three groups: guselkumab 100mg every four weeks (n=128), guselkumab 100mg every eight weeks (n=127), or placebo (n=126). The primary endpoint of the study was ACR20 at 24 weeks, which was achieved by 59 percent of the guselkumab four-week group, 52 percent of the eight-week group, and 22 percent of placebo patients. Serious adverse events were reported in four percent of placebo patients, three percent of the guselkumab eight-week group, and none of the guselkumab four-week group.48 Guselkumab, like other IL-23 inhibitors, is also effective against cutaneous psoriasis to a greater extent than TNF-alpha inhibitors. ACR20 results over 24 weeks showed the greatest improvement toward the end of the study rather than at the outset.
Filgotinib, a JAK1 inhibitor, is approved for treating PsA in some countries but not in the United States. The EQUATOR trial, a multicenter placebo-controlled 16-week study of filgotinib 200mg enrolled 131 patients with PsA and/or plaque psoriasis with an inadequate response to at least one conventional synthetic disease-modifying drug (csDMARD).49 The endpoint was ACR20, which was achieved at 16 weeks by 80 percent of the filgotinib patients compared to 33 percent of the placebo patients (p<0.0001). Adverse events were similar with 57 percent of filgotinib and 59 percent of placebo patients experiencing at least one treatment-emergent adverse event.49
Another JAK1 specific inhibitor, upadacitinib was evaluated in the 24-week SELECT-PsA-1 randomized placebo-controlled clinical study.50 This trial compared upadacitinib to the active agent adalimumab, a TNF-alpha inhibitor. Patients with PsA and prior inadequate response or intolerance to at least one non-biologic DMARD (N=1,704) were randomized to receive oral upadacitinib 15 or 30mg once a day, subcutaneous adalimumab 40mg every other week, or placebo. At 12 weeks, 70.6 percent of patients taking upadacitinib 15mg, 78.5 percent of patients taking upadacitinib 30mg, 64.8 percent of patients taking adalimumab, and 36.2 percent of placebo patients had achieved ARC20 (p<0.001 for both upadacitinib groups compared to placebo). At 12 weeks, both doses of upadacitinib were non-inferior to adalimumab and the 30-mg upadacitinib dose could be considered superior to adalimumab. PASI 75 was achieved at 12 weeks by 53 percent of adalimumab patients, 21 percent of placebo patients, and 63 percent and 62 percent, respectively, for upadacitinib 15 and 30mg patients. Adverse events occurred more frequently in the upadacitinib patients than placebo patients with 9.1 percent of upadacitinib 15mg and 12.3 percent of upadacitinib 30mg patients developing hepatic disorders.50 Laboratory monitoring is required for such patients.
As the number of specifically targeted drugs increase, it is important to recognize the types of agents at our disposal, their cytokine as well as non-cytokine targets, and the appropriate drugs for a variety of related diseases.41 Certain drugs, such as TNF-alpha inhibitors, work across a variety of domains of PsA, while other agents are more specific. However, as more is elucidated about these various agents, a clearer picture will emerge.
Pharmacoeconomic data suggest that PsA patients start incurring increased healthcare expenses a year or two before they are diagnosed with PsA.51 This may be further complicated by the fact that people with PsA, as a group, have significantly lower incomes than the general population. Although these data come from an older study, the importance of early diagnosis and prompt treatment of PsA remains relevant. While a subset of cutaneous psoriasis patients will go on to develop PsA, most of them will eventually develop osteoarthritis (OA), and it can sometimes be challenging to differentiate OA from PsA.
One important area to advance the diagnosis and effective treatment of PsA remains finding ways to stratify the risk of PsA in patients with cutaneous psoriasis. If it could be reliably determined which psoriasis patients were at highest risk, it might be possible to intervene earlier, which would likely improve outcomes.52
Topical Therapy for Psoriasis: What's New?
Based on a presentation by Linda Stein Gold, MD, Director of Clinical Research at the Henry Ford Health System in Detroit, Michigan
Topical treatments have been and continue to be a mainstay of psoriasis care. Despite their familiarity, there are many new developments to share, and some established products are worthy of review. Below are important pearls for psoriasis practice with respect to topical treatment options. Dr. Stein Gold presented a useful assortment of tips for optimizing the use of topicals in the treatment of psoriasis.
Tip 1: Vehicle matters. Vehicle can affect everything from product potency, penetration, and treatment adherence. A good example of how the vehicle can impact potency involves betamethasone dipropionate 0.05%, which can be categorized as a Class I, II, III, or V product depending on the vehicle. Diprosone lotion 0.05% is a Class V product, while diprolene cream and diprolene ointment 0.05% are Class I products.53 In these products, the active ingredient and its potency are the same, but changing the vehicle alters the drug potency in a substantial way.
However, it is not always possible to predict what sort of potency an active agent in a topical product will have based on the vehicle alone. It is not always intuitively evident. For instance, 0.05% betamethasone dipropionate in a spray is a Class III steroid, but desoximetasone 0.25% in a spray is a Class I steroid.
Tip 2: Vehicle still matters. Designer vehicles can further enhance certain active agents and result in better drugs. These elegant vehicles often meet with high levels of patient satisfaction. Cream vehicles, essentially oil-and-water formulations, are considered to be the sort of cosmetically desirable formulations that patients prefer, but the amount of surfactants needed to stabilize this type of formulation can impede drug penetration. This may be counteracted with PAD™ technology, which creates a robust droplet of oil, stabilized by encapsulation in an aqueous film. The result is the active agent is more soluble and achieves greater penetration than would otherwise be possible in a cream vehicle. In a Phase III clinical trial, MC2-01 cream with PAD™ technology was compared to both an active comparator and the MC2-01 cream vehicle alone. The active comparator in this study was commercially available calcitriol/betamethasone dipropionate (C/BD) in topical suspension. The study was conducted over eight weeks and included adults with mild to moderate psoriasis. The study endpoint was the percentage of patients achieving PGA treatment success. The MC2-01 PAD™ technology formulation was more effective than both the comparator and the vehicle, with differences emerging at Week 1 and achieving significance at four weeks. In addition, MC2-01 provided better reduction of itchiness compared to the vehicle. Adverse events were fewer than one percent in all groups.54
Tip 3: Proactive treatment does maintain results. Once patients have been treated to clear or almost clear, dermatologists might be uncertain if they should continue the same treatment, rotate to a different maintenance treatment, or have a drug holiday. In a study based on a four-week, open-label lead-in with a 52-week, randomized, double-blind maintenance phase of C/BD, 545 patients with psoriasis achieved PGA of "clear" or "almost clear" representing at least a two-grade improvement over baseline; they were then randomized to a proactive maintenance study arm (n=272) or a reactive maintenance study arm (n=273).55 The proactive group used C/BD foam and the reactive group used the foam vehicle alone twice a week with rescue C/BD foam available once a day for up to four weeks for flares. The primary endpoint of this study was the time to first relapse defined by PGA as "mild" or greater. A total of 46 percent (n=251) of patients completed the trial with time to first relapse 30 days in the reactive group compared to 56 days in the proactive group. Proactive patients had 41 more days in remission compared to reactive patients (p<0.001) and proactive patients had fewer relapses in a year (3.1 vs. 4.8).55
Tip 4: Combination therapy is generally better in psoriasis. Fixed-dose combination products for plaque psoriasis have demonstrated effectiveness, such as halobetasol propionate plus tazarotene lotion. The product is a fixed-dose combination of halobetasol 0.05% cream and 0.1% tazarotene cream in a polymeric emulsion. The combination product had improved delivery of the ingredients with better and more enhanced penetration than the monotherapeutic use of either agent alone. Enhanced penetration of the active agents translates into improved efficacy. Most patients reported favorable impressions of the lotion which provided safe, effective topical treatment of psoriasis.56
Synergistic drug combinations may be described as those where the effectiveness of the combined ingredients is greater than the sum of the parts. Additive drug combinations offer the effectiveness of two drugs combined.57 Not all drug combinations are synergistic, but many fixed-dose combination products are based on known synergistic pairings of drugs. Furthermore, optimal combinations and vehicles can further improve effectiveness. See Figure 1.

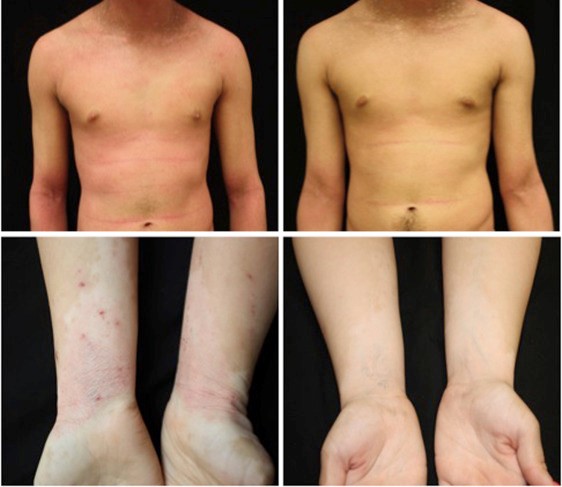
Results from two Phase III studies of the efficacy of halobetasol propionate and tazarotene lotion (0.01% and 0.045%, respectively) showed a two-grade IGA improvement over baseline at eight weeks.58 Photographic documentation of improvement appears in Figure 2.

Further, a recent open-label study of the long-term safety and efficacy of halobetasol propionate 0.01% and tazarotene 0.045% lotion for 555 patients with moderate to severe psoriasis found that most treatment-emergent adverse events were mild to moderate and decreased over time.59 Of the patients who participated for the full duration of the 52-week study, 77.5 percent maintained a BSA of less than or equal to five percent on treatment. The most common adverse events were application-site dermatitis, pruritus, pain, and irritation.59 In terms of efficacy, 54 percent of patients achieved "clear" or "almost clear" status at eight weeks, with rates improving over time; "clear" or "almost clear" was reported by 100 percent of patients by Week 32.59 When treatment was discontinued, the time elapsed before recurrence of symptoms was measured. Notably, 6.6 percent of patients did not require retreatment for one year and 20 percent of patients did not require retreatment for 12 weeks or more. Over 50 percent of patients could stop treatment for four or more weeks before needing to resume therapy.59
Tip 5: Layering drugs can affect efficacy. While layering drugs can, in some instances, improve efficacy, the question naturally arises as to why patients cannot do this on their own, simply applying one product on top of another. For instance, rather than using the fixed-dose combination product of halobetasol propionate and tazarotene, it would appear that the patient could apply each product individually. However, layering drugs—even drugs that work synergistically with each other—may not provide the same degree of efficacy as a fixed-dose combination product. For instance, the penetration of one or both drugs may be impeded rather than facilitated by layering one on top of the other. The cumulative response may be lessened in ways that are not readily predictable.
Tip 6: "Clear" maintains response better than "almost clear." In a post-hoc analysis of long-term management of moderate-to-severe psoriasis following discontinuation of halobetasol propionate 0.01% and tazarotene 0.045% fixed-dose combination lotion, 56 patients achieved "clear" status before the drug was ceased. Patients who achieved "clear" status did not require retreatment as quickly as those who achieved "almost clear." Thus, it is important to try to have the patient reach "clear" status before stopping the drug not only for clinical benefit but to prolong the period the patient can go before retreatment is necessary.60
Tip 7: We have great new molecules on the horizon. Tapinarof is a new small molecule that is a first-in-class agent. It acts as an aryl hydrocarbon receptor (AhR), which has shown effectiveness in treating psoriasis and atopic dermatitis, although its mechanism of action remains to be more fully elucidated.61 This novel molecule works inside the cell by repairing the skin barrier and reducing T-helper (Th)17 and Th2 cytokines, which decreases inflammation, which, in turn, increases antioxidant activity, thus reducing oxidative stress.62 Two Phase III clinical trials recently reported results. Patients were included if they were adults, had a PGA score greater than or equal to 2, and BSA greater than or equal to 3% and less than or equal to 20%. In these two companion double-blind controlled trials, patients were randomized to receive tapinarof 1% topical treatment once daily or vehicle with the primary endpoint of PGA response (clear=0 and almost clear=1) and at least a two-grade improvement at Week 12.63 In the two studies, 35 to 40 percent of patients got to "clear" or "almost clear" in 12 weeks compared to about six percent of vehicle-only patients.63 The most frequently reported treatment-emergent adverse events were folliculitis (about 20%), contact dermatitis, headache, and pruritis.63 Tapinarof was well tolerated, even in sensitive areas of the body.
Tip 8: Not all PDE4 inhibitors are the same. One might wonder: why do we need another phosphodiesterase-4 (PDE4) inhibitor? Roflumilast cream has been studied for mild to severe psoriasis and it is 50 to 300 times as potent as the other well-known PDE4 inhibitors in dermatology, crisaborole and apremilast. The oral formulation of roflumilast has been approved for treating chronic obstructive pulmonary disorder and a new cream formulation is being evaluated for its potential role in treating psoriasis. Two companion Phase IIb studies evaluated roflumilast cream in two concentrations, 0.3% and 0.15% creams. Both agents were statistically significantly superior to vehicle alone at Week 6 and these results persisted to the end of the 12-week study. The agents were effective in terms of clearance, reducing pruritus, and relieving disease burden. There was significant improvement in intertriginous psoriasis as early as Week 2 and most of those patients were "clear" by Week 8.64 The roflumilast cream was well tolerated. A foam formulation of roflumilast has entered study for at least mild scalp and body psoriasis. Early results indicate it was significantly more effective in clearing psoriasis than vehicle only and was well tolerated.
Bottom line. Topical treatments have been and likely long will remain the cornerstone of psoriasis therapy. Between new agents on the horizon and established treatments, there is a wealth of products from which to choose. Fixed-dose combination products and newer products may be particularly effective.
Implications of the COVID-19 Pandemic for the Management of Psoriasis
Based on a presentation by Joel M. Gelfand, MD, MSCE, Professor of Dermatology and Epidemiology, Vice Chair Clinical Research, Director of Psoriasis and Phototherapy Treatment Center at University of Pennsylvania Perelman School of Medicine
COVID-19 is usually a mild, self-limiting disease but about 15 percent of those infected will experience a life-threatening course with a case-fatality rate of approximately two percent, and which is higher in older than younger people.65,66 The most prominent and severe manifestation of COVID-19 infection is acute respiratory distress syndrome (ARDS) but other potentially life-threatening symptoms can also occur, including thromboembolic events.67 Key determinants in infection trajectory have emerged as age, sex, race/ethnicity, and comorbidities.68 Many of the comorbidities associated with psoriasis drive poor outcomes with COVID-19, such as obesity, diabetes, and cardiovascular disease. Moreover, the risk for poor outcomes increases when more than one comorbidity is present. Thus, one may assume that many psoriasis patients who contract the COVID-19 infection are at an elevated risk for worse outcomes given the frequent presence of comorbidities proven to result in worse COVID-19 outcomes.
Severe cases of COVID-19 are characterized by a persistent type 1 interferon response and increased levels of many pro-inflammatory cytokines, such as TNF, IL-1, IL-4, IL-6, IL-12, IL-13, and IL-17.68,69 The innate immune response launches earlier and the adaptive immune response occurs later. Immune suppression early in the disease can be more problematic in that it might cause the patient to be more at risk for uncontrolled infection with SARS-CoV-2.
During the first weeks of the pandemic, dermatologists recognized that there were sparse data to guide care with respect to respiratory infections for patients taking many typical psoriasis drugs, such as IL-17, IL-23, or TNF inhibitors. Since COVID-19 is a prototypical respiratory tract infection, this information was urgently needed, but progress was slowed by the fact that there are about a dozen different terms for respiratory tract infection found in safety databases. In a meta-estimate conducted from a variety of Phase III psoriasis trials combining these many different terms, there was special interest in three agents in particular, namely IL-17 (OR 1.56, p=0.03), IL-23 (OR 1.24, p=0.07), and TNF (OR 1.08, p=0.55) antagonists.70 Dr. Gelfand noted that the relevance of these findings to COVID-19 is uncertain and should not impact clinical decision making at this time. However, these data also emphasize the importance of more careful study of the impact of psoriasis treatment on respiratory infections.
Dermatologists were also concerned about the effects of biologics in patients who contract COVID-19. The data available to help formulate an answer to this important question were found in registries of spontaneous case reports, data from clinic-based cohorts that compared their data to the general population, and automated databases derived from electronic medical records.71 See Table 3.

A clinic-based cohort from a single center (n=1,193) was the only study in the literature that reported that patients on biologics were more likely to contract the SARS-CoV-2 infection (OR 3.43, range 2.25-5.73) and be hospitalized (OR 3.59, 1.49-8.63).77 By contrast, other studies did not show this association;78–80 for example, a multicenter study of 6,501 psoriasis patients on biologics found patients were not at heightened risk for COVID-19 hospitalization or death.81
Automated databases and large medical record systems were also searched. A primary-care database in the United Kingdom with over 17 million records found that people with autoimmune diseases, such as psoriasis, lupus, or rheumatoid arthritis, had a higher risk of COVID-19 mortality (HR 1.19, 1.11–1.27).82 The TriNetX global electronic medical records database includes 53 million people (91% in the United States) and 32,000 patients with COVID-19 diagnoses. Of this population, 214 had taken a TNF inhibitor or methotrexate within one year of being diagnosed with COVID-19. There appears to be no increased risk for such patients for developing COVID-19. The risk ratio for those taking both a TNF blocker plus methotrexate was 0.91 (0.68–1.22), while those taking methotrexate monotherapy had a risk ratio of 0.87 (0.62–1.23) and those taking TNF blockade as monotherapy had a risk ratio of 0.73 (0.47–1.14).83
In summary, the existing literature on how biologic therapy in patients with psoriasis might affect their risk of contracting COVID-19 or having poor outcomes with the infection is limited, but what is known is reassuring. Large-scale, long-term, population-based studies are needed with comparator groups and adjustments for confounding variables.
The National Psoriasis Foundation Task Force has issued guidance to help dermatologists and their patients balance the known benefits from various psoriasis treatments against theoretical risks. There was a high consensus on the Task Force that psoriasis patients who did not have COVID-19 should continue their biologic of oral therapies.71 For patients with psoriasis or psoriatic arthritis who had not yet started treatment but were considering it, individual patient factors have to be weighed. Patients with comorbidities associated with worse COVID-19 outcomes, those 65 years or older, those with relatively mild disease, and those at elevated risk for contracting COVID-19 might wish to consider delaying treatment or stopping ongoing treatment; while younger patients with more severe disease, no comorbidities, and at low risk of becoming infected may wish to start or continue their treatment. This decision must be individualized for each patient. By the same token, it must be recognized that not treating psoriasis or psoriatic arthritis poses risks to the patients' physical and emotional health, in particular psoriatic arthritis, which, if left untreated, can result in irreversible joint damage and disability.71
The National Psoriasis Foundation Task Force recommends that people with psoriatic disease and not otherwise contraindicated for the vaccine should take the vaccine. Systemic psoriasis treatments are not a contraindication for an mRNA-based COVID-19 vaccine (Pfizer or Moderna vaccines).71 Psoriasis patients who take the vaccine should in most cases continue their biologic or oral therapies for psoriasis.71
Dermatologists and other medical experts have made dramatic progress to manage this new SARS-CoV-2. While the pandemic has posed many challenges to clinicians and their patients, it has also underscored how well and efficiently the medical community has been able to work together and make progress and how willing patients have been to respond to the global needs of keeping each other safe.
Conclusion
This year was a challenging one on many fronts, but advances in the treatment of psoriasis patients continues. New molecules, improvements in topical therapy, and advances in the treatment of PsA patients continue. The National Psoriasis Foundation Task Force has been proactive in sorting out questions about what the pandemic means for patients with psoriasis and, in general, found patients in good health who do not have the COVID-19 infection may benefit from continuing biologic or oral treatments. More data are needed to understand the risks of psoriasis patients for respiratory infections, which are prevalent and not just in the time of this pandemic.
References
- Van Voorhees AS, Stein Gold L, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: Results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83(1):96–103.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498.
- Bimekizumab phase 3 data shows superior skin clearance over Humira in moderate-to-severe psoriasis patients [press release]. Brussels, Belgium: CISION, October 31, 2020 2020.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375(4):345–356.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286.
- Paller AS, Seyger MMB, Alejandro Magariños G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231–241.
- Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis [news release]. Basel, Switzerland. Novartis. June 1, 2021. https://www.novartis.com/news/media-releases/novartis-cosentyx-receives-fda-approval-treatment-children-and-adolescents-moderate-severe-plaque-psoriasis. Accessed July 1, 2021.
- Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938–947.
- No DJ, Inkeles MS, Amin M, Wu JJ. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatolog Treat. 2018;29(5):460–466.
- Rosenberg V. FC02.04. Presented at: EADV Virtual Congress 2020. Oct 28, 2020–Nov 1, 2020.
- Lilly's mirikizumab superior to Cosentyx (secukinumab) in a phase 3 study for patients with moderate to severe plaque psoriasis [press release]. Indianapolis: CISION, July 17, 2020.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–315.
- Papp KA, Strober B, Augustin M, Calabro S, Londhe A, Chevrier M. PSOLAR: design, utility, and preliminary results of a prospective, international, disease-based registry of patients with psoriasis who are receiving, or are candidates for, conventional systemic treatments or biologic agents. J Drugs Dermatol. 2012;11(10):1210–1217.
- Griffiths C. E. M., Reich K, Gooderham M, et al. Long term safety of ixekizumab in patients with moderate-to-severe plaque psoriasis up to 5 years: pooled data from 16 clinical trials. European Academy of Dermatology and Venereology; 2020.
- Reich K, Vender R, Merola J, et al. Long-term safety of guselkumab in patients with moderate to severe plaque psoriasis through 4 years of continuous follow-up in the VOYAGE 1 & 2 trials. European Academy of Dermatology and Venerology; 2020.
- Papp K, Gordon K, Bachelez H, et al. Long-Term Safety of Risankizumab in Patients With Moderate-to-Severe Plaque Psoriasis: Results From Pooled Clinical Studies. European Academy of Dermatology and Venereology; 2020. P1389.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98.e84.
- Papp K, Gottlieb AB, Naldi L, et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14(7): 706–714.
- Kimball AB, Schenfeld J, Accortt NA, et al. Cohort study of malignancies and hospitalized infectious events in treated and untreated patients with psoriasis and a general population in the United States. Br J Dermatol. 2015;173(5):1183–1190.
- Reich K. Bimekizumab safety in patients with moderate to severe plaque psoriasis: Analysis of pooled data from phase 2 and 3 clinical trials. European Academy of Dermatology and Venereology; 2020. FC02.07.
- Thaci D, Piaserico S, Warren RB. Long-term efficacy and safety of tildrakizumab for moderate to severe psoriasis: pooled analyses of two randomised phase 3 clinical trials (reSURFACE 1 and reSURFACE 2) through 5 years. European Academy of Dermatology and Venereology; 2020. D3T03.3C.
- Bardazzi F, Starace M, Bruni F, et al. Nail Psoriasis: An Updated Review and Expert Opinion on Available Treatments, Including Biologics. Acta Derm Venereol. 2019;99(6):516–523.
- Mease P, Smolen J, Behren F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79:123–131.
- Reich K. Ixekizumab shows early and sustained resolution of nail psoriasis in patients with psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from a multicentre, randomised, open-label, rater-blinded study (SPIRIT-H2H). EADVirtual; October 29-31, 2020 2020. FC02.06.
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the Interleukin-36 Pathway for the Treatment of Generalized Pustular Psoriasis. N Engl J Med. 2019;380(10): 981–983.
- Strober B et al. Perspectives on generalized pustular psoriasis treatment in North American: survey results from dermatologists in the Corrona Psoriasis Registry. European Academy of Dermatology and Venereology; 2020. P0749.
- Papp K, Gordon K, Thaci D, et al. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N Engl J Med. 2018;379(14): 1313–1321.
- Tehlirian C, Peeva E, Kieras E, et al. Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: a phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatology. 2021;3(3): E204–E213.
- Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1): 117–122.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14–17.
- Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62(17):2447–2457.
- Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol. 2014;41(7):1357–1365.
- Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284.
- Sudol-Szopinska I, Matuszewska G, Kwiatkowska B, Pracon G. Diagnostic imaging of psoriatic arthritis. Part I: etiopathogenesis, classifications and radiographic features. J Ultrason. 2016;16(64):65–77.
- Frischknecht L, Vecellio M, Selmi C. The role of epigenetics and immunological imbalance in the etiopathogenesis of psoriasis and psoriatic arthritis. Ther Adv Musculoskelet Dis. 2019;11:1759720×19886505.
- Bravo A, Kavanaugh A. Bedside to bench: defining the immunopathogenesis of psoriatic arthritis. Nat Rev Rheumatol. 2019;15(11):645–656.
- Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis. 2019;78(9):1215–1219.
- Mease PJ, Gladman DD, Collier DH, et al. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase 3 Trial. Arthritis Rheumatol. 2019.
- Araujo EG, Englbrecht M, Hoepken S, et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: Results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum. 2019;48(4):632–637.
- Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–1319.
- McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–1505.
- Nash P, Ohson K, Walsh J, et al. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis. 2018;77(5):690–698.
- Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–1125.
- Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2367–2377.
- McInnes IB, Anderson JK, Magrey M, et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N Engl J Med. 2021;384(13): 1227–1239.
- Kristensen LE, Jørgensen TS, Christensen R, et al. Societal costs and patients' experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis. 2017;76(9):1495–1501.
- Chao R, Kavanaugh A. Psoriatic Arthritis: Newer and Older Therapies. Curr Rheumatol Rep. 2019;21(12):75.
- Zeichner JA, Lebwohl MG, Menter A, et al. Optimizing topical therapies for treating psoriasis: a consensus conference. Cutis. 2010;86(3 Suppl):5-31; quiz 32.
- Selmer J, Vestbjerg B, Praestegaard M, Stein Gold L. MC2-01 Cream has Improved Overall Psoriasis Treatment Efficacy Compared to Calcipotriene Plus Betamethasone Dipropionate Topical Suspension. Henry Ford Health System Scholarly Commons. Dermatology Meeting Abstracts Web site. https://scholarlycommons.henryford.com/dermatology_mtgabstracts/115/. Published 2019. Accessed April 19, 2021.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2020.
- Tanghetti EA, Stein Gold L, Del Rosso JQ, Lin T, Angel A, Pillai R. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2019:1–8.
- Strom MA, Mohan GC, Lio PA. Therapeutic Synergy, Neutrality, and Antagonism: Combination Treatment in Dermatology. J Drugs Dermatol. 2016;15(10):1203–1207.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: Results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79(2): 287–293.
- Lebwohl MG, Stein Gold L, Papp K, et al. Long-term safety and efficacy of a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis: phase 3 open-label study. J Eur Acad Dermatol Venereol. 2021. 1152–1160.
- Stein Gold L, Lebwohl M, Bhatia N, et al. (2020). Long-Term Management of Moderate-to-Severe Plaque Psoriasis: Maintenance of Treatment Success Following Cessation of Halobetasol Propionate 0.01%/Tazarotene 0.045% Lotion. SKIN The Journal of Cutaneous Medicine. 2020;4(6), S79.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J Invest Dermatol. 2017;137(10):2110–2119.
- Bissonnette R, Stein Gold L, Rubenstein DS, Tallman AM, Armstrong A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84(4):1059–1067.
- Lebwohl M. Tapinarof Cream 1% Once Daily for the Treatment of Plaque Psoriasis: Efficacy and Safety in Two Pivotal Phase 3 Trials. EADV Virtual Meeting. https://conferences.m3medical.com/eadv-2020/article/%20efficacious-non-steroidal-topical-for-psoriasis/. Published 2020. Accessed April 17, 2021.
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of Roflumilast Cream for Chronic Plaque Psoriasis. N Engl J Med. 2020;383(3):229–239.
- Yang W, Kandula S, Huynh M, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203–212.
- Yang S, Cao P, Du P, et al. Early estimation of the case fatality rate of COVID-19 in mainland China: a data-driven analysis. Ann Transl Med. 2020;8(4):128.
- Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin Thromb Hemost. 2020;46(7):763–771.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–793.
- Wu K, Corum J. Charting a COVID-19 Immune Response. New York Times. October 5, 2020. https://www.nytimes.com/interactive/2020/10/05/science/charting-a-covid-immune-response.html
- Syed MN, Shin DB, Wan MT, Winthrop KL, Gelfand JM. The risk of respiratory tract infections in patients with psoriasis treated with interleukin 23 pathway-inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(5):1523–1526.
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force Guidance for Management of Psoriatic Disease During the Pandemic: Version 1. J Am Acad Dermatol. 2020;83(6):1704–1716.
- Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866.
- Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70(4):725–732.
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159(2):481–491.e483.
- Mahil SK, Dand N, Mason KJ, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J Allergy Clin Immunol. 2021;147(1):60–71.
- Brenner EJ, Pigneur B, Focht G, et al. Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International Databases. Clin Gastroenterol Hepatol. 2021;19(2):394–396.e395.
- Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: Real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33(5):e13475.
- Talamonti M, Galluzzo M, Chiricozzi A, et al. Characteristic of chronic plaque psoriasis patients treated with biologics in Italy during the COVID-19 Pandemic: Risk analysis from the PSO-BIO-COVID observational study. Expert Opin Biol Ther. 2021;21(2):271–277.
- Brazzelli V, Isoletta E, Barak O, et al. Does therapy with biological drugs influence COVID-19 infection? Observational monocentric prevalence study on the clinical and epidemiological data of psoriatic patients treated with biological drugs or with topical drugs alone. Dermatol Ther. 2020;33(6):e14516.
- Baniandrés-Rodríguez O, Vilar-Alejo J, Rivera R, et al. Incidence of severe COVID-19 outcomes in psoriatic patients treated with systemic therapies during the pandemic: A Biobadaderm cohort analysis. J Am Acad Dermatol. 2021;84(2):513–517.
- Gisondi P, Facheris P, Dapavo P, et al. The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br J Dermatol. 2020;183(2): 373–374.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436.
- Yousaf A, Gayam S, Feldman S, Zinn Z, Kolodney M. Clinical outcomes of COVID-19 in patients taking tumor necrosis factor inhibitors or methotrexate: A multicenter research network study. J Am Acad Dermatol. 2021;84(1):70–75