Pyoderma Gangrenosum-Like Presentation of Herpetic Panniculitis in a Patient With Immunosuppression
Abstract and Introduction
Abstract
Introduction: This case of herpes virus–induced panniculitis originally diagnosed as pyoderma gangrenosum (PG) illustrates the need for a high index of suspicion for atypical causes of cutaneous ulcers in patients who are immunocompromised.
Case Report: A 79-year-old male presented with a 3-month history of a painless chronic ulcer on the left buttock that was refractory to antibiotic therapy and intralesional corticosteroid. The medical history was notable for diabetes mellitus type 2 and rheumatoid arthritis managed with long-term methotrexate and low-dose prednisone. Because the patient initially had a painful and enlarging skin ulcer after intralesional treatment with corticosteroids, an undermined and violaceous ulcer, and an autoimmune condition, PG was suspected at the initial evaluation. A subsequent skin biopsy to complete the workup confirmed the unexpected diagnosis of herpetic panniculitis. The patient was started on antiviral therapy; a prolonged therapeutic and suppressive dose was required. This case highlights the importance of skin biopsy in the diagnosis of chronic ulcers to rule out infectious etiologies. Maintaining a high index of suspicion for rare causes of cutaneous ulcers in the patient with immunosuppression is paramount.
Conclusions: Herpes virus infection is only one atypical cause of ulcerative nodules in the immunocompromised patient. Skin biopsy should be considered in the immunocompromised patient with presumed PG that is not responding to standard of care treatment.
Introduction
The nonspecific clinical presentation of panniculitides requires clinical pathological correlation to determine the etiology. Infection-induced panniculitis is often seen in the immunocompromised patient and presents as 1 or more fluctuant nodules that ulcerate on the lower extremities.[1] Infection with herpes virus usually presents as an easily distinguishable vesicular rash. In the patient with immunosuppression, however, herpes virus infection can manifest with uncommon clinical findings.[2] The 2 subtypes of infection-induced panniculitis are primary, that is, a direct infection into the fat tissue, and secondary, which occurs through hematogenous spread of the pathogen.[3,4] However, the presence of ulcerative nodules may also be suggestive of the presence of other underlying etiologies, such as medium-sized vasculitis and vasculopathies (eg, calciphylaxis), factitial etiology, or inflammatory ulcerations (eg, pyoderma gangrenosum [PG]).
Case Report
A 79-year-old male with a history of diabetes mellitus and rheumatoid arthritis undergoing treatment with methotrexate 15 mg per week and prednisone 7.5 mg per day presented with a 3-month history of a mostly nontender chronic ulcer on the left buttocks that was refractory to wound care and antibiotic therapy. Physical examination revealed a 1.4 cm × 2.7 cm deep skin ulcer with violaceous borders. Superficial bacterial wound cultures were negative. Ultrasonography revealed no fluid collections. According to the diagnostic criteria proposed by Su et al[5] for PG, the patient's progressive skin ulcer with irregular undermining and violaceous border qualified as major criteria and the history of an autoimmune condition qualified as a minor criterion. As the diagnosis of PG was suspected, the ulcer was treated with intralesional triamcinolone.
Two weeks later, the ulcer had not improved. Instead, it had become tender, increased in size, and developed accentuated, bizarre borders and surrounding small nodules suggestive of possible pathergy. To complete the workup of this atypical ulcer, a punch biopsy of the edge of the ulcer was done, which revealed extensive necrosis within the subcutis as well as a diffuse infiltrate of lymphocytes, neutrophils, plasma cells, and histiocytes suggestive of an infectious or inflammatory etiology. Staining for herpes simplex virus 1 and 2 highlighted many cells in areas of fat necrosis (Figure). Staining for varicella-zoster virus (human herpesvirus 3) was negative. Results of additional, special stains and microbial cultures for fungal (periodic acid-Schiff technique), bacterial (Gram stain), and acid-fast mycobacterial (Fite method) organisms were negative. These findings supported the diagnosis of herpetic panniculitis. Polymerase chain reaction testing of a subsequent herpetic flare on the anterior leg confirmed the diagnosis of herpes simplex virus 2. The patient had an unknown history of shingles and herpes complex infection.
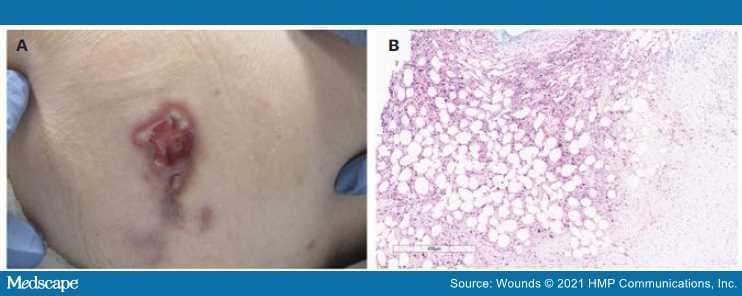
Figure.
(A) Ulcerative nodule with violaceous erythema, undermining, and slight elevation of borders with some fibrinous exudate on left hip. (B) High-power view of the subcutis demonstrating positive HSV 1/2 staining with extensive adipocyte necrosis.
HSV: herpes simplex virus
A 4-week course of valacyclovir 500 mg twice daily was started based on kidney function. The lesion on the left buttock began improving but had not completely resolved by 4-week follow-up. An infectious disease specialist was consulted, who recommended an extended course of valacyclovir 500 mg twice a day to complete up to 8 weeks of treatment, transitioning to prophylaxis dosing with valacyclovir 500 mg for 1 year. At the 3-month follow-up visit, the lesions were improved. Time to complete healing was approximately 6 months.
Discussion
This case is comparable to the only previously published case (to the authors' knowledge) of herpes virus leading to panniculitis, including that both patients received immunosuppressive therapy.[6] In the patient reported on by Tomasini and Ribero,[6] however, the ulcer completely regressed after 1 month of treatment with acyclovir. In comparison, although the patient in this case had shown some improvement with after 4 weeks of valacyclovir 500 mg daily, new lesions continued to develop, which prompted an increased dose to 1000 mg daily. This patient began showing improvement of all ulcers after a 10-day course of the increased dose.
Importantly, the patient in this case was initially and erroneously given a clinical diagnosis of PG; subsequent pathology results led to the final diagnosis of herpetic panniculitis. Pyoderma gangrenosum is a rare condition that commonly presents as a full-thickness ulcer with bluish purple undermining borders and can have white cell debris that looks like pus but often shows no growth on culture.[7] There have been prior reports of herpes virus infection mimicking PG.[8–12] However, none of these cases resulted in panniculitis. Distinguishing early PG from panniculitis can be challenging in clinical practice.[13]
Pyoderma gangrenosum is a clinical diagnosis arrived at only after infection, malignancy, and trauma have been ruled out.[14] The pathology of PG is nonspecific, but biopsies can help rule out similar clinical presentations, such as vasculitides and malignant abnormalities.[14] Other causes of cutaneous ulcers that may mimic PG include arterial and venous disease, vascular occlusion, hematologic causes, calciphylaxis, drug-induced ulceration, hypertension (Martorell ulcer), and inflammatory disorders such as cutaneous Crohn's disease.[15]
This case provides another example of herpes virus induced panniculitis in an immunocompromised patient and highlights a not uncommon clinical scenario of misdiagnosis of an atypical ulcer. The chance for misdiagnosis also highlights the importance of performing biopsy on all patients with presumed PG and maintaining a high index of suspicion for rare causes of cutaneous ulcers in those who are immunocompromised. Additionally, this case suggests that not all cases of herpes virus induced panniculitis resolve quickly even when treated with appropriate antivirals and that long term and prophylactic treatment may be indicated in some cases.
Limitations
Given that this is a single case study, the strength of the conclusions is limited. Several limitations led to an initial misdiagnosis of PG and, ultimately, a delay in appropriate treatment for herpetic panniculitis. The patient's autoimmune disease coupled with the wound morphology, for example, the violaceous wound border, was suggestive of PG according to the Su et al[5] criteria. The diagnosis of PG was made prior to performing immunohistochemical staining and obtaining tissue culture results. However, given the incidence of PG (3 to 10 patients per million population per year) and the rarity of herpetic panniculitis, empiric treatment for PG was warranted.[16] The lesions of the patient reported herein drastically worsened when treated empirically with intralesional triamcinolone, which prompted further workup described in the case.
Conclusions
Atypical presentations of herpes virus can manifest in patients who are immunocompromised and may result in infection-induced panniculitis. Additionally, skin biopsies should be considered in patients with chronic ulcers (especially in such patients who are immunocompromised) that are not responsive to standard of care treatment to rule out ulcerative conditions of infectious etiology. Treatment should consist of antiviral therapy that is active against herpes virus. Long-term treatment may be needed.
References
Bolognia JL, Schaffer JV, Duncan KO, Ko CJ. Dermatology Essentials. Elsevier Saunders; 2014.
Johnson R, Dover J. Cutaneous manifestations of human immunodeficiency virus. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, eds. Dermatology in General Medicine. 4th ed. McGraw-Hill; 1993:2637.
Morrison LK, Rapini R, Willison CB, Tyring S. Infection and panniculitis. Dermatol Ther. 2010;23(4):328–340. doi:10.1111/j.1529–8019.2010.01333.x
Johansson L, Thulin P, Low DE, Norrby-Teglund A. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin Infect Dis. 2010;51(1):58–65. doi:10.1086/653116
Su WPD, Davis MDP, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
Tomasini CF, Ribero S. Herpes infection: from skin to panniculitis involvement. G Ital Dermatol Venereol. 2019;154(2):224–226. doi:10.23736/S0392-0488.17.05700–5
Pompeo MQ. Pyoderma gangrenosum: recognition and management. Wounds. 2016;28(1):7–13.
Lau H, Lee ACW, Tang SK. Isolated foot ulcer complicating acute leukemia: an unusual manifestation of herpes simplex virus infection simulating pyoderma gangrenosum. Pediatr Hematol Oncol. 2003;20(6):477–480.
Kumar LS, Shanmugasekar C, Lakshmi C, Srinivas CR. Herpes misdiagnosed as pyoderma gangrenosum. Indian J Sex Transm Dis AIDS. 2009;30(2):106–108. doi:10.4103/0253-7184.62768
Brown TS, Callen JP. Atypical presentation of herpes simplex virus in a patient with chronic lymphocytic leukemia. Cutis. 1999;64(2):123–125.
Wahba A, Cohen HA. Herpes simplex virus isolation from pyoderma gangrenosum lesions in a patient with chronic lymphatic leukemia. Dermatologica. 1979;158(5):373–378. doi:10.1159/000250783
Saunderson RB, Tng V, Watson A, Scurry J. Perianal herpes simplex virus infection misdiagnosed with pyoderma gangrenosum: case of the month from the Case Consultation Committee of the International Society for the Study of Vulvovaginal Disease. J Low Genit Tract Dis. 2016;20(2):e14–e15. doi:10.1097/LGT.0000000000000178
Mortazavi H, Soori T, Azizpour A, Goodarzi A, Nikoo A. Photoclinic. Infection-induced panniculitis. Arch Iran Med. 2014;17(9):649–650.
Reichel S, Kushner J, LaFond AA. Nonhealing lower leg ulcers. JAMA Dermatol. 2017;153(1):81–82. doi:10.1001/jamadermatol.2016.3400
George C, Deroide F, Rustin M. Pyoderma gangrenosum - a guide to diagnosis and management. Clin Med (Lond). 2019;19(3):224–228. doi:10.7861/clinmedicine.19-3-224
Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008–1017. doi:10.1111/j.1468–3083.2009.03199.x
Wounds. 2021;33(5):E39-E41. © 2021 HMP Communications, LLC
Cite this: Pyoderma Gangrenosum-Like Presentation of Herpetic Panniculitis in a Patient With Immunosuppression - Medscape - May 01, 2021.

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home