Pustular Psoriasis
IL-36 and generalized pustular psoriasis: L'chaim!

By Warren R. Heymann, MD, FAAD
March 9, 2022
Vol. 4, No. 10
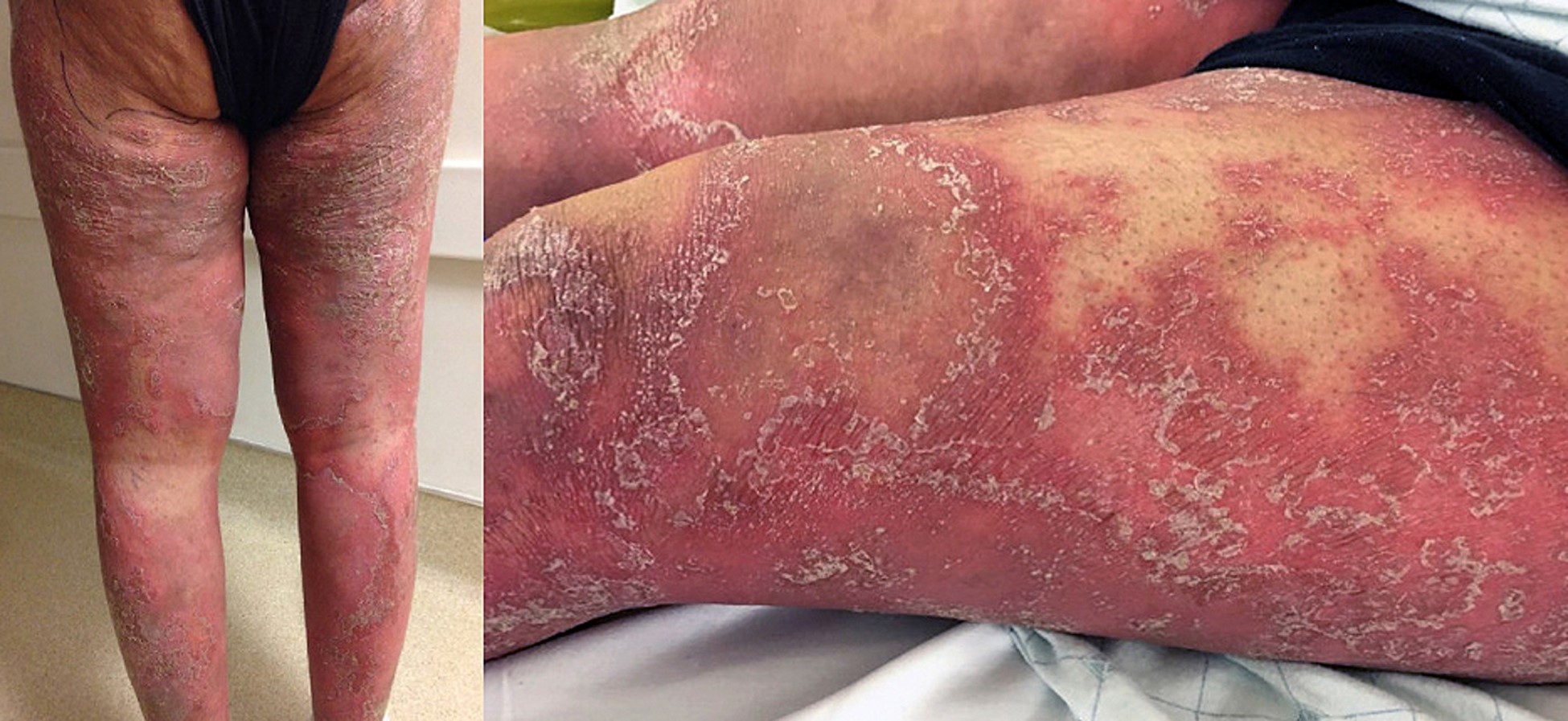
Pustular psoriasis may be subdivided into variants. Localized forms include palmoplantar psoriasis and acrodermatitis continua of Hallopeau. GPP includes pustular psoriasis of pregnancy (impetigo herpetiformis), exanthematic (an acute pustular eruption without systemic symptoms that resolves in days), annular (with pustules along the advancing edge), and the von Zumbusch subtype (a diffuse, generalized pustular eruption associated with systemic symptoms, such as fever, joint pains, headaches, and leukocytosis). The von Zumbusch subtype has a higher risk of mortality, especially if there are associated comorbidities such as hepatic failure, renal insufficiency, or cancer. Complications include hypocalcemia, septicemia, hyperthermia, liver damage, acute renal failure, and malnutrition. (2)
As Cowen eloquently asserts in his editorial "It is time to focus on pustular psoriasis," there is no FDA-approved treatment for either localized or GPP. Fortunately, our understanding of GPP has taken a quantum leap forward over the past decade. (3) Cowen states that in 2009, a severe, neonatal-onset form of pustular psoriasis with systemic inflammation termed deficiency of the IL-1 receptor antagonist, or DIRA (OMIM 612852) was described. These children respond quickly and dramatically to treatment with recombinant IL-1 receptor antagonist therapy such as anakinra. (3,4) Two years later, an analogous form of variable-age–onset pustular psoriasis (known as DITRA) caused by deficiency of the IL-36 receptor antagonist, IL-36Ra, which is encoded by the IL36RN gene (OMIM 614204) was detailed. "Subsequent sequencing studies have demonstrated that variants in IL36RN may be responsible for 19% to 41% of GPP and have also linked pathogenic variants in CARD14, AP1S3, SERPINA3, and myeloperoxidase with GPP. These discoveries have prompted a renewed effort to disentangle the pathogenesis of pustular psoriasis from plaque disease and, more specifically, to clarify the role of IL-1 family member cytokines (IL-1, IL-36) in the management of idiopathic pustular psoriasis, which still represents the majority of pustular psoriasis cases." (3)
The following excerpt from the abstract by Madonna et al offers a concise, lucid summation of the significance of IL-36 in psoriasis: "Psoriasis is an immune-mediated inflammatory skin disease that involves mainly T helper (Th)17, Th1 and Th22 lymphocytes, which cause hyper-proliferation of the epidermis with aberrant differentiation of keratinocytes, and local production of chemokines and cytokines. These fuel a self-amplifying loop where these products act on T cells to perpetuate cutaneous inflammatory processes. Among the various inflammatory mediators involved, interleukin (IL)-36 cytokines are important for the recruitment and activation of neutrophils and Th17 cells in psoriatic skin. More specifically, IL-36s induce chemokines and cytokines that interfere with differentiation/cornification programs in the epidermis, as well as promote pathological angiogenesis and endothelial cell activation. IL-36 cytokines belong to the IL-1 family, and comprise IL-36α, IL-36β, and IL-36γ agonists as well as IL-36 receptor antagonist and IL-38 antagonists. IL-36 cytokines are up-regulated in psoriatic epidermis, and their expression is strongly induced by TNF-α and IL-17. Contrarily, IL-38 antagonist is downregulated, and its impaired expression may be relevant to the dysregulated inflammatory processes induced by IL-36." (5)
Noe et al performed a retrospective longitudinal case series of 95 patients with GPP. The authors found that 70.5% of patients were women with a mean age of 50.3 years. On initial presentation, more than one-third of patients were hospitalized, and two-thirds were treated with more than 20 systemic therapies (antibiotics, antiviral, antifungals, systemic steroids, immunosuppressives, apremilast, biologics, and phototherapy). Over time, 35.8% of patients reported hospitalizations for disease flares. (6)
Spesolimab is a humanized anti–interleukin-36 receptor monoclonal antibody, being studied for the treatment of GPP flares. In a phase 2 trial, 53 randomly assigned patients with a GPP flare in a 2:1 ratio received a single 900-mg intravenous dose of spesolimab (35 patients) or placebo (18 patients). Seven patients (5 in the spesolimab group and 2 in the placebo group) had IL36RN mutations. Patients in both groups could receive an open-label dose of spesolimab on day 8, an open-label dose of spesolimab as a rescue medication after day 8, or both and were followed to week 12. At baseline, 46% of the patients in the spesolimab group and 39% of those in the placebo group had a Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore of 3, and 37% and 33%, respectively, had a pustulation subscore of 4. At the end of week 1, a total of 19 of 35 patients (54%) in the spesolimab group had a pustulation subscore of 0, as compared with 1 of 18 patients (6%) in the placebo group (P<0.001). A total of 15 of 35 patients (43%) had a GPPGA total score of 0 or 1, as compared with 2 of 18 patients (11%) in the placebo group (P=0.02). Drug reactions were reported in 2 patients (1 with DRESS syndrome). Among patients assigned to the spesolimab group, infections occurred in 6 of 35 (17%) through the first week; among patients who received spesolimab at any time in the trial, infections had occurred in 24 of 51 (47%) at week 12 (the most reported being urinary tract infection in 3 patients and influenza in 3 patients). Antidrug antibodies were detected in 23 of 50 patients (46%) who received at least one dose of spesolimab. The authors concluded that this phase 2 randomized trial of spesolimab resulted in a higher incidence of lesional clearance of GPP at 1 week than placebo but was associated with infections and systemic drug reactions. Longer and larger trials are warranted to determine the effectiveness and risks of spesolimab in patients with pustular psoriasis. They also note that the long-term administration of spesolimab is being investigated with a subcutaneous formulation to prevent flares of GPP (Effisayil 2 trial). (7) (The reader should note that the spesolimab study was funded by Boehringer Ingelheim, as are the Effisayil trials. I have no conflicts of interest — financial or otherwise — regarding this study.)
The potential severity of the von Zumbusch variant of GPP may warrant a greater risk-to-benefit ratio than other forms of pustular psoriasis. In Jewish numerology (gematria), the number 18 ("chai") means life (think of "L'chaim — to life!" from Fiddler on the Roof) — 36 is twice life. Perhaps advances in anti-IL-36 directed therapy will also mean two lives for patients with GPP — their former life of affliction replaced with a new one of disease control.
Point to Remember: IL-36 plays an important role in the pathogenesis of pustular psoriasis. Targeting this cytokine therapeutically may have the capability to alter the course of this potentially life-threatening disease. Further studies are necessary to determine the risk/benefit ratio of this approach.
Our experts' viewpoints
Joel Gelfand, MD, MSCE, FAAD
James J. Leyden Professor of Dermatology and of Epidemiology
Vice Chair of Clinical Research and Medical Director, Dermatology Clinical Studies Unit
Director, Psoriasis and Phototherapy Treatment Center
University of Pennsylvania Perelman School of Medicine
GPP is a severe life-threating skin disease but despite the serious nature of this condition there have been no large-scale detailed epidemiological studies of this disease in the U.S., with the last major study on this topic occurring in 1991 at a single institution. (8) We recently published a 20 U.S. center epidemiological study and concluded the following (6):
GPP is quite rare in the U.S. For example, five study sites (25%) did not have 5 GPP cases during the 10-year study period. This finding is striking as all sites were academic referral centers.
More than 20 different systemic therapies have been tried in the U.S. demonstrating that none have risen to the top as being consistently effective, and thus there is significant unmet medical need.
GPP is more common in women (70%) and the disease is highly burdensome. At presentation more than a third of patients were hospitalized and over time 36% of patients were hospitalized for a flare of GPP. Interestingly, women had a decreased risk of an emergency department or hospital encounter (odds ratio, 0.19; 95% CI, 0.04-0.83) for a GPP flare. This observation is a new finding which requires replication.
Mark Lebwohl, MD, FAAD
Dean of Clinical Therapeutics
Professor of Dermatology
Kimberly and Eric J. Waldman Department of Dermatology
Icahn School of Medicine at Mount Sinai
Despite breakthroughs in psoriasis, treatment of generalized pustular psoriasis (GPP) remains a major challenge. The skin of affected patients with GPP loses many of its functions. As a result, patients can come in febrile or hypothermic because of the loss of temperature control; hypotension and renal failure can occur because of loss of fluid through the skin; hypoalbuminemia frequently occurs because of loss of protein through the skin; patients develop a microcytic anemia because of loss of iron; and electrolyte imbalances such as hypocalcemia are common. Patients often develop lower limb edema as a result of high output cardiac failure, and the most common cause of death is sepsis because of loss of the skin's protective barrier against bacteria. As a result, GPP remains a life-threatening condition. Treatments in the past have been too slow and insufficiently effective.
There are no approved therapies for GPP in the United States. While systemic steroids are still commonly used in patients with GPP, the evidence is clear that use of systemic steroids for this condition is associated with increased fatality. In a large study by Ryan and Baker, nearly a quarter of patients treated with systemic steroids for GPP died, most often as a consequence of the steroid therapy. (9) Retinoids, methotrexate, cyclosporine, and TNF blockade can be effective but are often too slow for this life-threatening condition. Newer biologics have recently been approved outside the U.S., but the endpoints in studies leading to their approval demonstrate how difficult GPP is to treat. In some studies, the definition of treatment success is any improvement at all. Moreover, studies still use PASI scores even though induration, a key component of the PASI score, is not present in GPP; and those studies do not evaluate the presence or absence of pustules, a key element of GPP. Spesolimab thus represents a major breakthrough in the treatment of pustular psoriasis. It targets the specific cytokine known to drive GPP, IL-36, and it works quickly and in a high proportion of treated patients.
Dr. Gelfand had disclosed financial relationships with the following to the AAD at the time of publication: Abcentra, Amgen, BMS, Boehringer Ingelheim, Celgene Corporation, Daavlin Company, Dr. Reddy, Eli Lilly and Company, FIDE, GlaxoSmithKline, Happify, Healio, Janssen Pharmaceuticals, Inc, NEMA Research, Inc, Neumentum, Inc., Neuroderm LTD, Novartis Pharmaceuticals Corp., Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi US Services, Society for Investigative Dermatology, Sun Pharmaceutical Industries Ltd., Trevi Therapeutics, UCB, vTv Therapeutics.
Dr. Lebwohl had disclosed financial relationships with the following to the AAD at the time of publication: AbbVie, Aditum Bio, Allergan, Inc., Almirall, AltruBio Inc., Amgen, AnaptysBio, Arcutis, Inc., Arena Pharmaceuticals, Aristea Therapeutics, Arrive Technologies, Avotres, Inc., BiomX Ltd., BirchBioMed, BMD Skincare, Inc., Boehringer Ingelheim, Brickell Biotech, Inc., Cara Therapeutics, Castle Biosciences, Inc, Corrona, Inc., Bristol-Myers Squibb, Dermavant Sciences, Dr. Reddy, Eli Lilly and Company, EMD Serono, Evelo Biosciences, Inc., Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research & Education of Dermatology, Helsinn Healthcare, Hexima Ltd., Incyte Corporation, Inozyme Pharma, Janssen Research & Development, LLC, Kyowa Kirin, Leo Pharma Inc, LEO Pharma, US, Meiji Seika Pharma Co., Ltd, Menlo Therapeutics, Mindera, Mitsibushi Pharma, Neuroderm LTD, Ortho Dermatologics, Pfizer Inc., Regeneron, Seanergy Maritime Holdings Corp., Theravance Biopharma, UCB, Verrica Pharmaceuticals Inc.
Full disclosure information is available at coi.aad.org.
Ring J, Locher W. Leo Ritter von Zumbusch. In Pantheon of Dermatology, Löser C, Plewig G, Burgdorf WHC (eds). Springer, 2013, pp 1222-1232.
Shah M, Al Aboud DM, Crane JS, Kumar S. Pustular Psoriasis. 2021 Aug 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 30725687.
Cowen EW. It Is Time to Focus on Pustular Psoriasis. JAMA Dermatol. 2021 Dec 8. doi: 10.1001/jamadermatol.2021.4634. Epub ahead of print. PMID: 34878490.
Cowen EW, Goldbach-Mansky R. DIRA, DITRA, and new insights into pathways of skin inflammation: what's in a name? Arch Dermatol. 2012 Mar;148(3):381-4. doi: 10.1001/archdermatol.2011.3014. PMID: 22431779; PMCID: PMC3464919.
Madonna S, Girolomoni G, Dinarello CA, Albanesi C. The Significance of IL-36 Hyperactivation and IL-36R Targeting in Psoriasis. Int J Mol Sci. 2019 Jul 5;20(13):3318. doi: 10.3390/ijms20133318. PMID: 31284527; PMCID: PMC6650959.
Noe MH, Wan MT, Mostaghimi A, Gelfand JM and the Pustular Psoriasis in the US Research Group. Evaluation of a Case Series of Patients With Generalized Pustular Psoriasis in the United States. JAMA Dermatol. 2021 Dec 8. doi: 10.1001/jamadermatol.2021.4640. Epub ahead of print. PMID: 34878491.
Bachelez H, Choon SE, Marrakchi S, Burden AD, Tsai TF, Morita A, Navarini AA, Zheng M, Xu J, Turki H, Anadkat MJ, Rajeswari S, Hua H, Vulcu SD, Hall D, Tetzlaff K, Thoma C, Lebwohl MG; Effisayil 1 Trial Investigators. Trial of Spesolimab for Generalized Pustular Psoriasis. N Engl J Med. 2021 Dec 23;385(26):2431-2440. doi: 10.1056/NEJMoa2111563. PMID: 34936739.
Zelickson BD, Muller SA. Generalized Pustular Psoriasis: A Review of 63 Cases. Arch Dermatol. 1991;127(9):1339–1345. doi:10.1001/archderm.1991.01680080075005
Ryan TJ, Baker H. Systemic corticosteroids and folic acid antagonists in the treatment of generalized pustular psoriasis. Evaluation and prognosis based on the study of 104 cases. Br J Dermatol. 1969 Feb;81(2):134-45. doi: 10.1111/j.1365-2133.1969.tb15995.x. PMID: 4304162.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
posted by dermatica at
March 17, 2022
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home