Published in Dermatology News · April 29, 2022 Annual Costs Estimated for Wart Treatment in the U.S. for 2015-2017

WEDNESDAY, April 27, 2022 (HealthDay News) -- The estimated annual costs were $846 million for cutaneous warts for 2019 in the United States, and costs for anogenital warts were $127 million, according to a research letter published online April 27 in JAMA Dermatology.
Ronald Berna, from the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, and colleagues evaluated annual health care utilization and costs associated with common cutaneous and anogenital wart treatments in a retrospective cohort study using the deidentified Optum Clinformatics Database.
On average, for each year from 2017 through 2019, 263,070 individuals with cutaneous warts (mean age, 44 years; 52 percent female) and 28,516 with anogenital warts (mean age, 43 years; 40.7 percent female) were captured. The researchers found that 64.2, 1.8, and 2.2 percent of individuals with cutaneous warts were treated with lesional destruction, intralesional injection, and prescription topical medications, respectively. For anogenital warts, 35.3, 0.6, and 16.5 percent were treated with lesional destruction, intralesional injection, and prescription topical medications, respectively. For 2019, the annual costs were estimated at $846 million and $127 million for cutaneous and anogenital warts, respectively. The per-patient costs were estimated at $288.28 and $431.47 for cutaneous and anogenital warts, respectively.
"Understanding health care utilization for warts provides useful information for anticipatory guidance (e.g., expected number of treatments) and insight into the burden of disease," the authors write.
Skin Care Physicians of Costa Rica
Clinica Victoria en San Pedro: 4000-1054
Momentum Escazu: 2101-9574
Please excuse the shortness of this message, as it has been sent from
a mobile device.


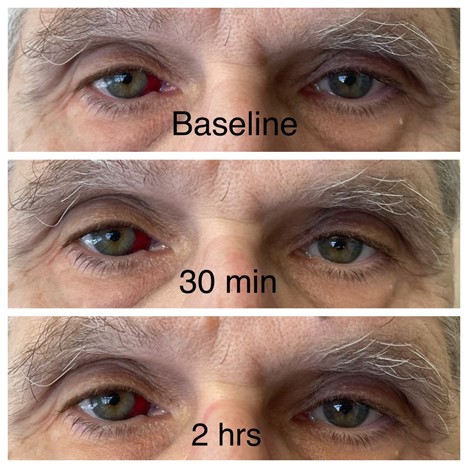
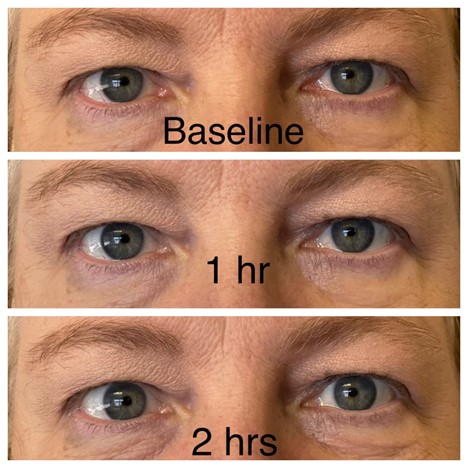

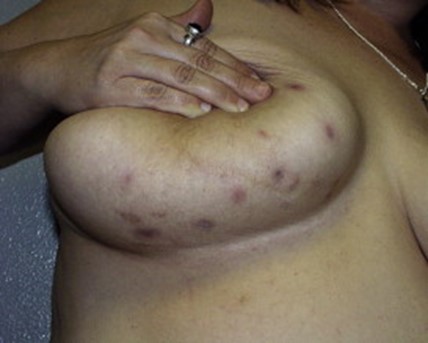




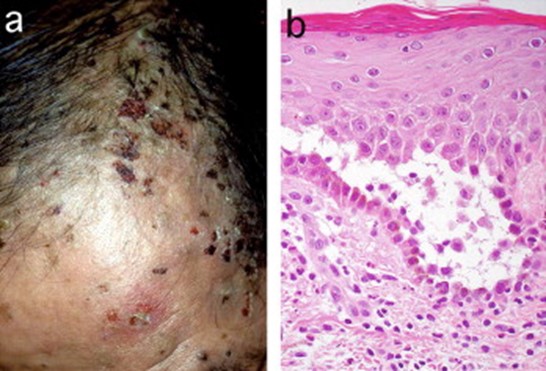 JAAD 2005; 52: 839-845.
JAAD 2005; 52: 839-845.
Finasteride and sexual dysfunction
Years ago, after an episode of urinary tract infection, I went to see a urologist for, well, you do not want to know, because they do unspeakable things to guys who have urinary tract infections. Anyway, the urologist was also a specialist in managing sexual dysfunction. I was discussing with him how there is very little overlap between dermatology and urology except for the use of finasteride, which we use for alopecia. He said to me with an air of of surprise in his voice, "You use finasteride? Aren't you concerned about the sexual dysfunction side effects?"
At the time, I thought, well, he specializes in sexual dysfunction; so, he probably sees people all day who claim they have sexual dysfunction from finasteride and does not see the ones who do not have that side effect. His perception would be horribly biased by his practice of selecting patients who had a problem. I still thought that there is probably no relationship between finasteride and sexual dysfunction.
Nguyen et al's article describing a signal for sexual dysfunction from finasteride based on spontaneous reports still leaves me feeling as if there is no real signal. If finasteride did cause sexual dysfunction, almost certainly it would do it more with higher doses than with low doses, and I suspect that it would be extraordinarily unlikely for anybody who had sexual dysfunction and was on minoxidil to blame the minoxidil. Therefore, it seems entirely reasonable to me that there would be relatively more reports of sexual dysfunction from finasteride than from minoxidil. I do not see any basis for the authors conclusion, "it is unlikely that confounding factors alone account for the totality of the signal observed–there is likely a true underlying effect albeit not as significant as observed." On the contrary, it seems that confounding factors and reporting biases alone would account for the so-called "signal" that was observed.
It is easy to draw mistaken conclusions from spontaneous reporting of data. That said, when starting patients on finasteride for alopecia, I would warn them of reports of sexual dysfunction. Educating patients about this risk may make the risk more likely (given the nocebo effect), but that is something we probably have to live with, given the potential repercussions of not warning someone who may develop sexual dysfunction.