LON late onset neutropenia from rituximab...
EARLY RECOGNITION OF RITUXIMAB-INDUCED LATE ONSET NEUTROPENIA IS ESSENTIAL

By Warren R. Heymann, MD, FAAD
April 13, 2022
Vol. 4, No. 15
 Rituximab is an anti-CD20 chimeric antibody directed against a surface transmembrane protein marker (CD20) expressed on B-cells during differentiation from pre B-cell until the plasma cell stage, involved in the maturation and activation of B cells. Approved in 1998, rituximab's off-label use has been expanding beyond its other FDA-approved indications (CD-20 positive B-cell non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, microscopic polyangiitis, and granulomatosis with polyangiitis). The list of off-label uses of rituximab continues to increase exponentially; from a dermatological perspective, its use in bullous pemphigoid, dermatomyositis, systemic lupus erythematosus, and refractory graft-versus-host disease are just a few examples where the drug may be impactful. (1)
Rituximab is an anti-CD20 chimeric antibody directed against a surface transmembrane protein marker (CD20) expressed on B-cells during differentiation from pre B-cell until the plasma cell stage, involved in the maturation and activation of B cells. Approved in 1998, rituximab's off-label use has been expanding beyond its other FDA-approved indications (CD-20 positive B-cell non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, microscopic polyangiitis, and granulomatosis with polyangiitis). The list of off-label uses of rituximab continues to increase exponentially; from a dermatological perspective, its use in bullous pemphigoid, dermatomyositis, systemic lupus erythematosus, and refractory graft-versus-host disease are just a few examples where the drug may be impactful. (1)Rituximab's well-recognized adverse events include infusion reactions, lymphopenia, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. (2) Early-onset neutropenia is extremely rare, with only a handful of cases reported to date. (3) Rituximab-induced late-onset neutropenia (R-LON), although underrecognized, has been increasingly reported. According to the National Cancer Institute common toxicity criteria, R-LON is defined as unexplained grade 3 or more neutropenia (<1.0×103/μL) occurring at least 3–4 weeks after the rituximab infusion. (4) Other authors define R-LON as (<1.5×103/μL) after at least 4 weeks — the end point is not well-defined, being reported as late as 2 years following treatment.
Increasingly, extended courses of rituximab are being utilized to keep patients in remission. Rashid et al have demonstrated how maintenance infusions have prevented relapses of pemphigus. (5) According to Hosp et al, "LON is not infrequent. Its incidence is estimated at approximately 6.0% but might vary within a broad range (1.3% –29.9%). The risk of LON possibly depends on the underlying disease, and is supposed to be highest in coexisting lupus erythematosus. Comedication with disease-modifying antirheumatic drugs does not seem to influence its incidence." (6) Zonozi et al identified 107 episodes of LON in 71 patients. The cumulative incidence at 1 year was 6.6% (the incidence rate in the first year was higher compared to thereafter). Systemic lupus erythematosus and combination therapy with cyclophosphamide were each independently associated with an increased risk of LON. (7)
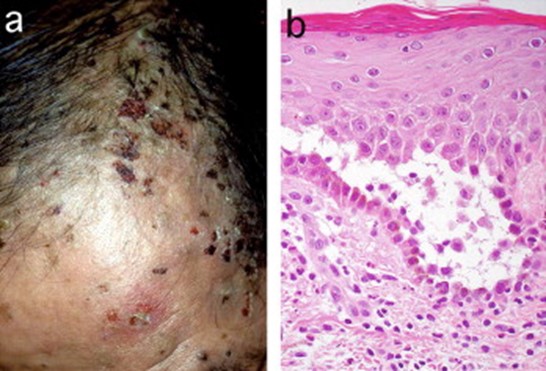 JAAD 2005; 52: 839-845.
JAAD 2005; 52: 839-845.Is R-LON clinically significant? In their review, Hosp et al observed that although patients are frequently asymptomatic, severe infectious complications, including fatalities, have occurred. Administration of filgastrim (G-CSF) has been demonstrated to shorten time to recovery but did not affect overall outcomes. (6) Zonozi et al reported that fever and sepsis complicated 31.3% and 8.5% of episodes, respectively. Most patients (69%) were treated with filgrastim. Rituximab rechallenge occurred in 87% of patients, of whom 21% developed recurrent LON. (7) Boch et al identified LON in 5 of 117 rituximab-treated pemphigus patients compared to none of 15 patients who did not receive the drug. The R-LON in their patients was short-lived; none required specific therapy (antibiotics, filgastrim) or hospitalization. (8)
The pathomechanism(s) of R-LON remain to be determined. Hypotheses include the production of antineutrophil antibodies, expansion of large granular lymphocyte cells and aberrant B-cell reconstitution, and the association of an FcgRIIIa polymorphism. (2) Immunodysregulation during B-cell recovery coinciding with excess levels of B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) may be at play. (6) The presumption is that B-cell depletion leads to alterations in hematopoietic growth factors that promote lymphopoiesis and downregulate granulopoiesis. (7)
More will be learned about R-LON with increasing experience and study. Dermatologists must be aware of this adverse reaction of rituximab and monitor patients accordingly. While most patients will be fine, that is not a guarantee, and vigilance in recognizing severe infection is mandatory. As the sage Yogi Berra eloquently stated: "It gets late early out there."
Point to Remember: Late-onset neutropenia has been increasingly recognized in patients receiving rituximab. Although usually short-lived and without clinical consequence, severe infections and fatalities have been reported. Dermatologists need to be cognizant of this adverse reaction and monitor patients carefully during their course of rituximab therapy.
Our expert's viewpoint
Robert A. Somer, MD
Professor of Medicine, Cooper Medical School of Rowan University
Head, Division of Hematology and Medical Oncology; Executive Director, Clinical Trials Program
MD Anderson Cancer Center at Cooper
Dermatologists must be aware of the risks of rituximab, as use of this agent increases, not only for the treatment of pemphigus vulgaris, but other dermatologic conditions as mentioned above. As use of rituximab expands to treat multiple autoimmune disorders, sub-specialists will need to remain cognizant of the risks and benefits of this agent and monitor CBCs not only during the course of treatment but also thereafter. LON is much more common than we initially thought, and often found incidentally. Patients with pemphigus vulgaris may be at higher risk of cellulitis compared to other indications, if they experience rituximab associated LON. Luckily, most cases reverse and are unlikely to require therapy. If severe infections or sepsis do exist, treatment with G-CSF may be necessary. As more dermatologists get comfortable prescribing this agent for infusion, they must remain vigilant in monitoring. More investigation is certainly needed to accurately define the mechanism of LON which can then lead to prevention and treatment strategies as rituximab use continues to increase.
Hanif N, Anwer F. Rituximab. 2020 Nov 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
Sahu KK, Petrou N, Cohn Z, Khanna S. Rituximab-induced late-onset neutropenia. BMJ Case Rep. 2019 Dec 29;12(12):e233569.
Shah S, Kavadichanda CG, Belani P, Ganesh RN, Negi VS. Early onset neutropenia and thrombocytopenia following rituximab in lupus nephritis. Int J Rheum Dis. 2019 May;22(5):946-950.
Sahu KK, Petrou N, Cohn Z, Khanna S. Rituximab-induced late-onset neutropenia. BMJ Case Rep. 2019 Dec 29;12(12):e233569.
Rashid H, Lamberts A, van Maanen D, Bolling MC, Diercks GFH, Pas HH, Jonkman MF, Horváth B. The effectiveness of rituximab in pemphigus and the benefit of additional maintenance infusions: Daily practice data from a retrospective study. J Am Acad Dermatol. 2020 Nov;83(5):1503-1505.
Hosp C, Goebeler M, Benoit S, Stoevesandt J. Rituximab-mediated late onset neutropenia in autoimmune blistering diseases: negligible or under-estimated threat? J Dtsch Dermatol Ges. 2020 Oct;18(10):1162-1164.
Zonozi R, Wallace ZS, Laliberte K, Huizenga NR, Rosenthal JM, Rhee EP, Cortazar FB, Niles JL. Incidence, Clinical Features, and Outcomes of Late-onset Neutropenia from Rituximab for Autoimmune Disease. Arthritis Rheumatol. 2020 Sep 6. doi: 10.1002/art.41501. Epub ahead of print. PMID: 32892495.
Boch K, Zillikens D, Langan EA, Schmidt E, Ludwig RJ. Low prevalence of late-onset neutropenia after rituximab treatment in patients with pemphigus. J Am Acad Dermatol. 2020 Dec;83(6):1824-1825.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
posted by dermatica at
April 13, 2022
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home