HSP como inhibidores de la inflamación
Shocking news: Heat shock protein inhibition finally enters the realm of dermatologic therapy

By Warren R. Heymann, MD, FAAD
Oct. 23, 2024
Vol. 6, No. 43

Ben Abdallah et al. eloquently summarizes the biology of HSP. "Heat shock proteins (HSP) are a group of chaperone molecules whose major function is the maintenance of cellular homeostasis by folding and promoting the function of endogenous proteins (referred to as ''client proteins''). The heat shock protein 90 (HSP90) is the most abundant HSP (4-6% of total protein during cellular stress); it exists in two cytosolic isoforms (HSP90α and HSP90β) and two organelle-specific isoforms including glucose-regulated protein 94 (GRP94/gp96) localized in the endoplasmic reticulum and tumor necrosis factor receptor-associated protein 1 (TRAP1) localized in the mitochondria. HSP90 consists of three conserved domains: an N-terminal domain where the ATP-binding site is located (an ATPase activity is required for its chaperone function); a middle domain which is involved in binding to client proteins: and a C-terminal domain, which is responsible for HSP90 dimerization. Several co-chaperones (e.g., HSP70, CDC37, and AHA1) modulate the ATPase activity or functional range of client proteins. HSP90 is sometimes referred to as the 'signal transduction chaperone' given a large number of kinases and transcription factors being client proteins. Many of these client proteins are involved in key inflammatory pathways. Thus, HSP90 inhibition may target these client proteins involved in inflammation, providing a rationale as a treatment for multiple inflammatory skin conditions. HSP90 inhibition has been shown to mediate anti-inflammatory effects in inflammatory models such as rheumatoid arthritis and systemic lupus erythematosus…" RGRN-305 is an imidazopyridine derivative HSP90 inhibitor displaying a high affinity to the N-terminal ATP-binding site for HSP90α and HSP90β, and thereby blocks the ATPase activity and chaperone function." (2)
HSP90 is a downstream regulator of tumor necrosis factor (TNF-α) and interleukin (IL-17A) signaling and may serve as a novel target in the treatment of psoriasis. Bregnhøj et al. performed a phase Ib study evaluating the safety and efficacy of the HSP90 inhibitor (RGRN-305) for treating plaque psoriasis. The study was an open-label, single-arm, dose-selection, single-center proof-of-concept study. Patients with plaque psoriasis were treated with 250 mg or 500 mg RGRN-305 daily for 12 weeks. Efficacy was evaluated clinically using the Psoriasis Area and Severity Index (PASI), body surface area (BSA), Physician's Global Assessment (PGA) scores, and the Dermatology Life Quality Index (DLQI). Skin biopsies collected at baseline and at 4, 8, and 12 weeks after initiation of treatment were used for immunohistochemical staining and for gene expression analysis. Safety was monitored via laboratory tests, vital signs, electrocardiogram, and physical examinations. Six of the 11 patients who completed the study responded to RGRN-305 with a PASI improvement between 71% and 94%, whereas five patients were considered nonresponders with a PASI response < 50%. No severe adverse events were reported. Four of seven patients treated with 500 mg RGRN-305 daily experienced a mild-to-moderate exanthematous drug-induced eruption attributed to the drug. Two patients discontinued the study because of this exanthematous eruption. RGRN-305 treatment resulted in pronounced inhibition of the IL-23, TNF-α, and IL-17A signaling pathways and normalization of histological changes and psoriatic lesion gene expression profiles in patients who responded to treatment. The authors concluded that treatment with RGRN-305 showed acceptable safety, especially in the low-dose group, and was associated with clinically meaningful improvement in a subset of patients with plaque psoriasis, indicating that HSP90 may serve as a novel future target in psoriasis treatment. (3)
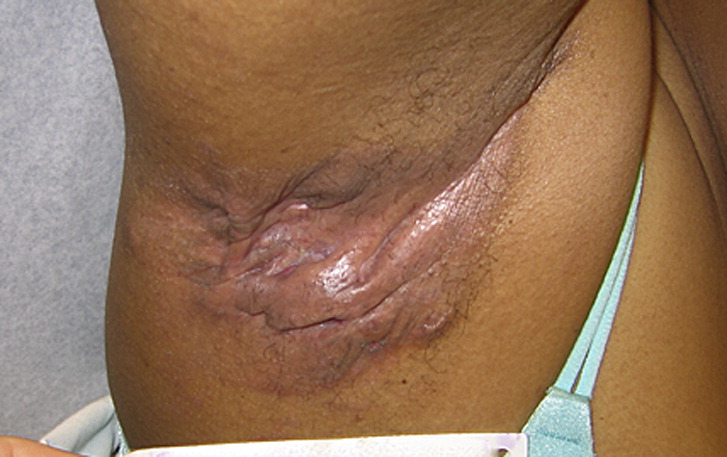
HSP90 inhibition has increasingly become a research focus in autoimmune diseases, including autoimmune bullous diseases. Preclinical rodent experiments demonstrated that HSP90 blockers ameliorate autoimmune encephalomyelitis, rheumatoid arthritis, and systemic lupus erythematosus. Tukaj et al. demonstrated that HSP90 is a promising treatment target in autoimmune bullous diseases including epidermolysis bullosa acquisita, bullous pemphigoid, and possibly dermatitis herpetiformis. (5)
The role of HSP in cancer has been a long-standing area of active inquiry. Magwenyane et al. state that the overexpression of HSP90 occurs in patients with cancer, triggering unstable harmful kinase functions, which enhance carcinogenesis. They discuss various computational models used to develop HSP90 inhibitors as anticancer agents. (6) Many clinical trials utilizing HSP inhibitors have been performed over recent decades. Pimitespib (Jeselhy®) is an oral small molecule inhibitor of the α and β isoforms of HSP90. In 2022, the drug was approved in Japan and China for treating of gastrointestinal stromal tumors. (7,8)
In the classic film Casablanca, Captain Renault (Claude Rains), a regular attendee at Rick's Café, is under order to shut down the joint — he uses the premise of gambling to do so, declaring "I'm shocked! Shocked to find that gambling is going on in here!" as the croupier responds, "Your winnings, sir." I am equally "shocked" that heat shock protein inhibitors have entered the realm of dermatologic therapy. Perhaps the heat will be turned up on researchers to deliver some positively shocking results.
Point to Remember: Heat shock protein inhibition is a novel approach that may prove valuable for dermatologists treating inflammatory, autoimmune, bullous, and oncologic diseases.
Our experts' viewpoint
Hakim Ben Abdallah, MD
Department of Dermatology and Venereology
Aarhus University Hospital
Lars Iversen MD, DMSc
Professor, Department of Dermatology and Venereology
Aarhus University Hospital
Heat shock protein 90 is a common protein that is involved in the cellular machinery that regulates the function of client proteins. Notably, multiple client proteins play essential roles in tumor cell proliferation and survival, hence HSP90 inhibition has been pursued as a therapeutic modality in cancer. (9,10) Approximately 20 different HSP90 inhibitors have entered cancer clinical trials, with the first HSP90 inhibitor (i.e., Pimitespib) receiving its approval in 2022 for gastrointestinal stromal tumors in Japan. (8) Besides the oncogenic client proteins, numerous client proteins are implicated in inflammation, though the research concerning HSP90 inhibition and inflammation is relatively sparse, especially clinical research.
Several preclinical studies have demonstrated that HSP90 inhibition alleviates inflammation in animal models within dermatology, gastroenterology, and rheumatology. These findings support the involvement of HSP90 in numerous inflammatory pathways, highlighting its potential as a drug target in inflammatory disorders.
The first clinical data of HSP90 inhibition in a skin disease was discovered by coincidence in a cancer trial with the oral HSP90 inhibitor RGRN-305. Surprisingly, during this study, a patient with cancer and concomitant severe plaque psoriasis covering > 40% of the skin surface experienced complete skin clearance following treatment with RGRN-305. Afterward, preclinical studies and the proof-of-concept clinical study confirmed the potential of HSP90 inhibition in plaque psoriasis, and the recent randomised placebo-controlled proof-of-concept study revealed the therapeutic potential of HSP90 inhibition in hidradenitis suppurativa. (3,4)
While the recent findings suggest HSP90 may be a novel drug target for inflammatory skin diseases, drug-related skin rashes have been reported, especially in psoriasis patients receiving 500 mg RGRN-305 once daily. (3) Indeed, further research is warranted to examine the optimal dosage regimen in skin diseases, which may differ between diseases, to enhance the efficacy while maintaining a favourable safety profile. Furthermore, given that HSP90 inhibitors usually are small molecules, topical delivery may also be a feasible route of administration, which remains to be evaluated in patients.
Four distinct HSP90 isoforms exist with different client proteins, but the inflammatory activity for each isoform remains unexplored. Thus, selective inactivation of these HSP90 isoforms may provide valuable insights that can be translated into potential therapeutic benefits.
Lastly, the effects of HSP90 inhibition are likely wide-ranging due to the targeting of hundreds of client proteins; thus, the therapeutic utility of HSP90 inhibition may extend beyond psoriasis and hidradenitis suppurativa to include other skin diseases, which hopefully may benefit patients.
De Maio A, Santoro MG, Tanguay RM, Hightower LE. Ferruccio Ritossa's scientific legacy 50 years after his discovery of the heat shock response: a new view of biology, a new society, and a new journal. Cell Stress Chaperones. 2012 Mar;17(2):139-43. doi: 10.1007/s12192-012-0320-z. Epub 2012 Jan 18. PMID: 22252402; PMCID: PMC3273555.
Ben Abdallah H, Seeler S, Bregnhøj A, Ghatnekar G, Kristensen LS, Iversen L, Johansen C. Heat shock protein 90 inhibitor RGRN-305 potently attenuates skin inflammation. Front Immunol. 2023 Feb 7;14:1128897. doi: 10.3389/fimmu.2023.1128897. PMID: 36825010; PMCID: PMC9941631.
Bregnhøj A, Thuesen KKH, Emmanuel T, Litman T, Grek CL, Ghatnekar GS, Johansen C, Iversen L. HSP90 inhibitor RGRN-305 for oral treatment of plaque-type psoriasis: efficacy, safety and biomarker results in an open-label proof-of-concept study. Br J Dermatol. 2022 May;186(5):861-874. doi: 10.1111/bjd.20880. Epub 2022 Mar 30. PMID: 34748646.
Ben Abdallah H, Bregnhøj A, Emmanuel T, Ghatnekar G, Johansen C, Iversen L. Efficacy and Safety of the Heat Shock Protein 90 Inhibitor RGRN-305 in Hidradenitis Suppurativa: A Parallel-Design Double-Blind Trial. JAMA Dermatol. 2024 Jan 1;160(1):63-70. doi: 10.1001/jamadermatol.2023.4800. PMID: 38055242; PMCID: PMC10701664.
Tukaj S, Zillikens D, Kasperkiewicz M. Heat shock protein 90: a pathophysiological factor and novel treatment target in autoimmune bullous skin diseases. Exp Dermatol. 2015 Aug;24(8):567-71. doi: 10.1111/exd.12760. Epub 2015 Jun 3. PMID: 25980533.
Magwenyane AM, Ugbaja SC, Amoako DG, Somboro AM, Khan RB, Kumalo HM. Heat Shock Protein 90 (HSP90) Inhibitors as Anticancer Medicines: A Review on the Computer-Aided Drug Discovery Approaches over the Past Five Years. Comput Math Methods Med. 2022 May 31;2022:2147763. doi: 10.1155/2022/2147763. PMID: 35685897; PMCID: PMC9173959.
Xie X, Zhang N, Li X, Huang H, Peng C, Huang W, Foster LJ, He G, Han B. Small-molecule dual inhibitors targeting heat shock protein 90 for cancer targeted therapy. Bioorg Chem. 2023 Oct;139:106721. doi: 10.1016/j.bioorg.2023.106721. Epub 2023 Jul 8. PMID: 37467620.
Hoy SM. Pimitespib: First Approval. Drugs. 2022 Sep;82(13):1413-1418. doi: 10.1007/s40265-022-01764-6. PMID: 35986838.
Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19(3):347-65.
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537-49.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
posted by dermatica at
October 24, 2024
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home