Miel, para preservar muestras para inmunofluoresencia...en vez de Solución de Michel.
Dermatopathology's taste of honey

By Warren R. Heymann, MD
September 2, 2020
Vol. 2, No. 34
Apparently, the answer is a resounding "yes!"
Despite the use of serological ELISA tests utilized for diagnosing autoimmune bullous disorders, DIF of perilesional skin remains the diagnostic gold standard, with sensitivities ranging from 82-91% and specificity of 98%. (1) For transport, saline-soaked gauze may be utilized, but the specimen must be delivered to the laboratory within 48 hours. Given the vagaries of specimen pick-up schedules in my practice, I am not comfortable with that time frame. (2)
The answer for this dilemma is sweet — as in honey. Honey has been utilized since antiquity as a binder vehicle, but also topically for medicinal and cosmetic purposes. Ancient Greeks and Egyptians used honey to treat skin wounds, burns, and infections. Persian traditional medicine documented the efficacy of honey for the treatment of eczema. It has also been utilized for tinea, seborrhea, dandruff, diaper dermatitis, psoriasis, hemorrhoids, and anal fissures. Honey is a bee‐derived, supersaturated solution composed mainly of fructose and glucose, and containing proteins and amino acids, vitamins, enzymes, minerals, and other minor components. Mechanisms of action include antioxidant activity, the induction of cytokines and matrix metalloproteinase expression, as well as epithelial‐mesenchymal transition in wounded epidermis. (3,4)
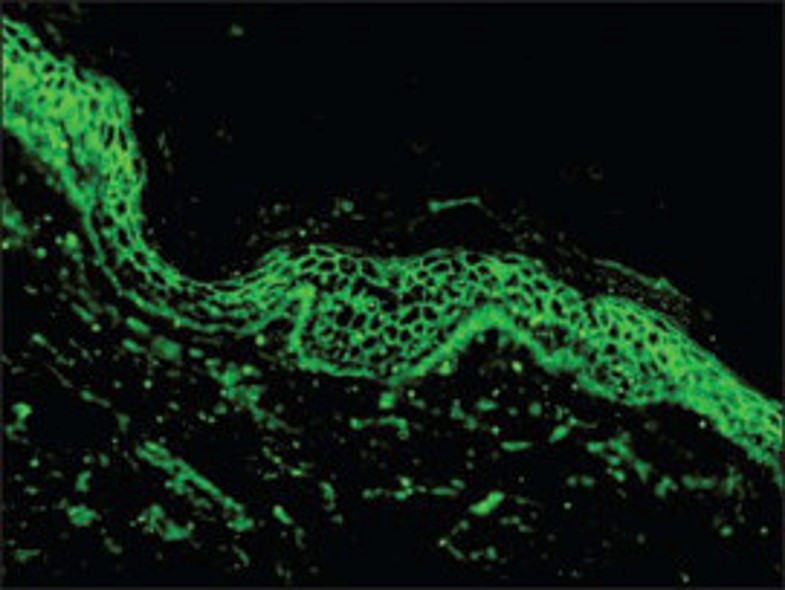
Rao et al, based on the centuries-old use of honey as a preservative, evaluated the use of honey as transport medium for DIF. A total of 36 snap‐frozen DIF-positive biopsy samples were divided into 3 groups of 12 specimens each. The biopsy specimens were placed in contact lens holding boxes and approximately 3 ml of honey was poured on top of the specimens. These samples were processed at the end of days 5, 10, and 20, respectively. The DIF was positive in all except for one case of dermatitis herpetiformis (DH) in the study. Limitations of this study were that fresh biopsy samples were not utilized and the efficacy of honey as DIF transport medium was not directly compared to MM. (5)
Kumudhini et al compared honey to MM as a transport medium for skin biopsy specimens used for DIF and antigen mapping. Group I consisted of 45 freshly‐taken skin specimens earmarked for DIF testing. It was divided into three groups (A, B, and C), each containing 15 specimens. Biopsy specimens were sliced into two, one each for MM and honey. Samples in group A were processed at the end of week 1 while those in group B and C were processed at the end of weeks 2 and 4, respectively. Group II consisted of five specimens of epidermolysis bullosa (EB) which was further divided into three groups; two specimens were processed for antigen mapping at the end of week 1, while others were processed at the end of week 2 (two specimens) and 4 (one specimen). Sensitivity of honey as a transport medium for skin biopsy specimens was 100%, 92.6%, and 53.8% at weeks 1, 2, and 4, respectively. The antigen mapping was positive in all specimens. The authors concluded that the utility of honey was comparable to MM for DIF samples tested at weeks 1 and 2 but was lower at week 4. If the samples transported in honey can reach the DIF laboratory within 2 weeks, the immunoreactants will be well-preserved for identification. (6)
In conclusion, if you run out of MM for your DIF specimens, go to the local market, grab some honey as transport medium, and send your specimen to the lab so it is received within two weeks. The only wrinkle in this otherwise sweet scenario is that there is a declining bumblebee population over the past decade attributable to habitat loss, changing climate, pathogen transmission, invasion of nonnative species, and pesticides. (7)
Point to Remember: Don't fret if you cannot find Michel's medium for your DIF specimen. A taste of honey will suffice!
Our expert's viewpoint
Kiran Motaparthi, MD
Preservation of immunoreactants for visualization during fluorescence microscopy requires flash or snap freezing. If freezing followed by cryosectioning is not possible directly after biopsy collection — a common scenario for dermatologists who are distant to a laboratory — a nonfixative transport medium should be used. (8) For up to 24 hours, normal saline is the optimal transport medium, given its low cost and higher specificity based on reduction of background fluorescence. Unfortunately, the sensitivity for immunoreactant detection decreases at 48 hours, limiting the use of saline in this context. (9) Specimens are technically viable in MM for up to 6 months (10), but for diagnostic utility, storage in MM for up to 2 weeks produces accurate immunofluorescence findings in 90% of cases. (11) As Dr. Heymann notes in his commentary, storage in honey for up to 2 weeks was recently found to deliver greater than 92% sensitivity for DIF, an impactful finding in settings where access to MM is limited by cost or availability. (12) In other words, if only 24 hours separates biopsy from freezing, normal saline is preferred. If transport requires between 1 and 14 days, either MM or honey should be used. Of these 3 media, honey is the most cost efficient, while MM is the most expensive. Importantly, if perilesional skin is inadvertently placed in formalin, the biopsy should be repeated. Autoantibodies cannot be detected in pemphigus and bullous pemphigoid after only 2 minutes and 10 minutes in formalin, respectively. (13)
Of interest to dermatopathologists who perform DIF, diagnostic immunofluorescence is consistently retained in frozen blocks for at least 4 months. (14) In DIF-positive slides stored at room temperature, a reliable diagnosis can still be made before 12 months. (15)
van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous disease. J Dtsch Dermatol Ges. 2018;16(9):1077-1091.
Elston DM, Stratman EJ, Miller SJ. Skin biopsy: Biopsy issues in specific diseases. J Am Acad Dermatol 2016; 74: 1-16.
McLoone P, Warnock M, Fyfe L. Honey: A realistic antimicrobial for disorders of the skin. J Microbiol Immunol Infect 2016; 49: 161-167.
Burlando B, Cornara L. Honey in dermatology and skin care: A review. J Cosmet Dermatol 2013; 12: 306-313.
Rao R, Jindal A, Bhogal B, Pai SB. Direct immunofluorescence microscopy of skin biopsy samples preserved in honey. J Am Acad Dermatol 2017; 76: 761-763.
Kumudhini S, Pai, Rao C, Rao R. A comparative study of Michel's medium versus honey as a transport medium versus honey as a transport medium for skin specimens prior to direct immunofluorescence microscopy and antigen mapping. J Cutan Pathol 2019; 46: 729-735.
Cameron SA, Sadd BM. Global trends in bumble bee health. Annu Rev Entomol 2019; Oct 14 [Epub ahead of print]
Patel AN, Simpson RC , Cohen SN. In a patient with an immunobullous disorder, is transportation of the skin biopsy in normal saline adequate for direct immunofluorescence analysis? A critically appraised topic. Br J Dermatol 2013;169:6-10.
Vodegel RM, de Jong MC, Meijer HJ, Weytingh MB, Pas HH , Jonkman MF. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol 2004;4:10.
Vaughan Jones SA, Salas J, McGrath JA, Palmer I, Bhogal GS , Black MM. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel's medium. Dermatology 1994;189 Suppl 1:131-2.
Nisengard RJ, Blaszczyk M, Chorzelski T , Beutner E. Immunofluorescence of biopsy specimens: comparison of methods of transportation. Arch Dermatol 1978;114:1329-32.
Kumudhini S, Pai S, Rao C , Rao R. A comparative study of Michel's medium versus honey as a transport medium for skin specimens prior to direct immunofluorescence microscopy and antigen mapping. J Cutan Pathol 2019;46:729-35.
Arbesman J, Grover R, Helm TN , Beutner EH. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol 2011;65:106-11.
Mackie RM, Young H , Campbell IA. Studies in cutaneous immunofluorescence. I. The effect of storage time on direct immunofluorescence of skin biopsies from bullous disease and lupus erythematosus. J Cutan Pathol 1980;7:236-43.
Dikicioglu E, Meteoglu I, Okyay P, Culhaci N , Kacar F. The reliability of long-term storage of direct immunofluorescent staining slides at room temperature. J Cutan Pathol 2003;30:430-6.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
posted by dermatica at
September 02, 2020
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home