Angiomatosis dermica difusa... angiomatosis reactiva tuve un caso recientemente biopsia Granuloma Piogeno
Honing in on diffuse dermal angiomatosis

By Warren R. Heymann, MD
February 17, 2021
Vol. 3, No. 7
Diffuse dermal angiomatosis (DDA) was conceptualized as a variant of reactive angioendothelimatosis (RA) in 1994 by Krell et al, reporting on two women, both with severe peripheral vascular disease requiring bypass grafts, presenting with ulcerated, violaceous plaques on the legs. The authors observed: "Unlike previously described cases of RA, our patients' lesions were due to a diffuse proliferation of endothelial cells in the reticular dermis with only minimal, focal proliferation of these cells. Positive immunostaining with antibodies to Factor VIII-related and CD34 antigens adds evidence that the proliferated cells in the dermis were endothelial cells." Both patients demonstrated complete clinical clearing after bypass grafts were placed suggesting that the inciting factor was vascular insufficiency. (1)
Aside from atherosclerosis, subsequent reports have demonstrated that DDA is associated with arteriovenous fistulae and patients with large, pendulous breasts (macromastia). My first introduction to DDA was a woman with ulcerations of her breasts — I recall that my clinical concern was either vasculitis or breast cancer. Hui et al have reported that DDA may mimic inflammatory breast carcinoma both clinically and on mammography. (2) Other entities entering the differential diagnosis of DDA include Kaposi sarcoma, acroangiodermatitis (pseudo-Kaposi sarcoma), angiosarcoma, (3) and any occlusive vasculopathy/vasculitis causing a retiform purpura. (4)
Other ischemic factors associated with DDA include smoking, cutis marmorata telangiectatica congenita, granulomatosis with polyangiitis, anticardiolipin antibodies, and calciphylaxis. (3) In their review of 24 cases of calciphylaxis, O'Connor et al identified seven cases of DDA. The only statistically significant associations in these cases were African-American race and congestive heart failure. (5)
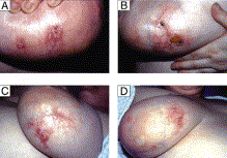 Image from reference 7.
Image from reference 7. Grossman-Kranseler et al provide a cogent conceptualization of the pathophysiology of DDA. The common denominator in the pathophysiology of DDA is ischemia causing hypoxia leading to increased VEGF (vascular endothelial growth factor) yielding increased vascular proliferation. "Hypoxia inducible factor (HIF-1) stabilization is critical to wound healing and angiogenesis following hind limb ischemia models. HIF-1 stimulates the stromal derived factor-1 (SDF-1)/CXCR-4 axis, which stimulates the proliferation and homing of CD34+endothelial progenitor cells. VEGF contributes to endothelial cell differentiation. Likely, the CD34+/VEGF+ spindle cell population represents an endothelial progenitor cell population. Further histologic/morphologic and immunohistochemical studies could be performed in cases of DDA to determine the cell-specific expression of SDF-1, CXCR-4, and HIF-1." (6)
Treatment of DDA revolves around improving blood flow by judicious consideration of any of the following: cessation of smoking, revascularization (bypass grafts, stents), and anticoagulation. Steroids and isotretinoin have been reported to be effective based on their ability to inhibit angiogenesis. McLaughlin et al reported the case of a 28-year-old woman with ulcerative breast DDA, who improved dramatically on isotretinoin (1 mg/kg for 2 months) with complete healing of the ulcers. (7)
There is much to be learned about the molecular aspects of hypoxia, wounds, and wound healing. VEGF elevation has also been observed in other entities such as von Hippel-Lindau disease and POEMS syndrome. Bevacizumab infusions have safely extended survival in a report of two patients with otherwise untreatable, life-threatening POEMS syndrome. (8) To the best of my knowledge, bevacizumab has not been utilized in patients with DDA. I suspect it would be valuable in recalcitrant cases unresponsive to the previously mentioned therapeutic options.
Point to Remember: Diffuse dermal angiomatosis reflects elevated VEGF in response to hypoxia. It should be in the differential diagnosis of ulcerated plaques in the context of retiform purpura, especially in those with vascular compromise. The prognosis is good if reconstitution of vascular perfusion can be accomplished — by surgical or medical means.
Our expert's viewpoint
Jack L. Arbiser, MD, PhD
Thomas J. Lawley Professor of Dermatology
Emory University School of Medicine
Grossman-Kranseler et al describe a case of diffuse dermal angiomatosis (DDA), a rare vascular proliferative disorder in an unusual location. Notably, they exclude infectious etiologies (HHV8) and demonstrate the presence of the angiogenic factor vascular endothelial growth factor (VEGF) in the lesions. They hypothesize that hypoxia induces hypoxia inducible factor 1 (HIF1) leading to vascular proliferation. We previously described a case of ulcerated DDA which occurred in pendulous breast tissue that responded to isotretinoin, which has antiangiogenic activities.
While DDA is a rare disease, it sheds light on more common disorders, characterized by endothelial proliferation and ulceration. While ulceration is instinctively thought to be a disorder of insufficient vascularization, it is usually characterized by excess endothelial proliferation. It would be more accurate to call most ulceration a disorder of inefficient vascularization. The endothelial proliferation and inflammation results in an environment hostile to re-epithelialization. DDA is usually associated with defects in large vessel perfusion (pendulous breasts, fistulas, etc.) but ulceration can be associated with small vessel perfusion defects (hemangioma of infancy, pyogenic granuloma, venous ulcers). More recently, we have identified novel therapeutic strategies to address ulcerative disorders. These include NADPH oxidase inhibitors such as gentian violet, which has been used on hemangiomas of infancy and most recently in a series of patients with pyogenic granuloma. We have identified another growth factor associated with excess vascular proliferation, Angiopoeitin like 4 (Angptl4), that is a major growth factor in hemangiomas, and that it can be blocked by R-propranolol, an isomer of propranolol that is present in commercial propranolol, but is not a beta blocker. It is likely that R-propranolol is the active principle of propranolol in hemangiomas, and that Angptl4 is likely a major pathogenic factor in vascular proliferative diseases of the skin, including DDA.
Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis : A variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol 1994; 21: 363-370.
Hui Y, Elco CP, Heini NF, Lourenco AP, et al. Diffuse dermal angiomatosis mimicking inflammatory breast carcinoma. Breast j 2018; 24: 196-198.
Touloei K, Tongdee E, Smirnov B, Nousari C. Diffuse dermal angiomatosis. Cutis 2019 ; 103 : 181-183.
Bhattacharya T, Sluzevich J. Generalized retiform purpura as a presenting sign of diffuse dermal angiomatosis. Dermatol Online J 2018; 24 (5): 10.
O'Connor HM, Wu Q, Lauzon SD, Forcucci JA. Diffuse dermal angiomatosis associated with calciphylaxis: A 5-year retrospective institutional review. J Cutan Pathol 2020; 47: 27-30.
Grossman-Kranseler J, Rhim E, Ruhoy S. Diffuse dermal angiomatosis: Report of a classic case with a comment on the pathophysiology based on the histologic findings. Am J Dermatopathol 2019 Dec 11 [Epub ahead of print].
McLaughlin ER, Morris R. Weiss SW, Arbiser JL. Diffuse dermal angiomatosis of the breast: Response to isotretinoin. J Am Acad Dermatol 2001; 45: 462-465.
Perfetti V, Palladini G, De Amici M, Livraghi L, et al. Bevacizumab treatment followed by maintenance in life-threatening POEMS syndrome. Ann Hematol 2013; 92: 1133-1134.
posted by dermatica at
February 19, 2021
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home