DO NOT GET BURNED BY MISSING STAPHYLOCOCCAL SCALDED SKIN SYNDROME

By Warren Heymann, MD
April 28, 2021
Vol. 3, No. 17
I would be surprised if any first-year dermatology resident could not differentiate Staphylococcal scalded skin syndrome (SSSS) from toxic epidermal necrolysis (TEN). What is straightforward now was a source of confusion for decades. The following is an elegant summation by Mockenhaupt et al of how dermatology has arrived at the current understanding of SSSS:
"Staphylococcal scalded skin syndrome (SSSS) is a rare, systemic blistering skin disorder. The clinical features were first described in 1878 by Baron Gottfried Ritter von Rittershain, who observed 297 cases among children in a single Czechoslovakian foundling asylum in a 10-y period Presumably in 1891 Staphylococcus aureus (S. aureus) was isolated from a patient with SSSS. The disease received little attention until Alan Lyell in 1956 described toxic epidermal necrolysis (TEN), a skin eruption resembling scalding of the skin. It soon became apparent that exfoliation associated with S. aureus infection occurred specifically in the zona granulosa of the epidermis, whereas the condition without association to bacteria showed splitting at the dermoepidermal junction. Based on these histological features, the former condition was renamed SSSS and the latter TEN. Nevertheless, the confusion persisted for decades, because both diseases were often referred to as 'Lyell's syndrome,' sometimes also called 'staphylococcal TEN or Lyell's syndrome 'versus drug TEN.' Involvement of toxins from S. aureus in SSSS was proved in 1970 after injection of sterile fluids obtained from intact bullae and from culture medium of phage II S. aureus into neonatal mice. Meanwhile, four different serotypes of exfoliative toxins named ETA, ETB, ETC, and ETD are identified. Biochemical, molecular biological, and crystallographical data of ETA and ETB indicate that both toxins are atypical glutamic acid-specific trypsin-like serine proteases, which have no significant binding to any part of the body apart from the skin. Therefore, wherever the toxin is released from a toxin-producing bacterial colony in the body, the toxin accumulates in the skin and causes disruption of desmosomes via proteolytic cleavage of desmoglein I." (1)
What can possibly be new about SSSS? Four aspects – 1) epidemiology; 2) causation; 3) the clinical spectrum; and 4) therapeutic approach.
Epidemiology
The incidence of SSSS seems to be increasing in recent years. The Nationwide Inpatient Sample 2008-2012 was analyzed, including a 20% sample of U.S. hospitalizations and 589 cases of SSSS. The mean annual incidence of SSSS was 7.67 per million U.S. children, with 45.1 cases per million U.S. infants age < 2 years. (2) Utilizing the same database, the annual incidence among U.S. adults was 0.98 cases per million. (3).
Causation
To date, the exfoliative toxins have been associated with only certain strains of S. aureus, most of which have been methicillin-susceptible S. aureus (MSSA). As noted above, strains causing SSSS were usually reported as belonging to phage group II. More recent studies have reported isolates belonging to sequence types (ST) 15, ST121, ST 2126, and ST2993, using multilocus sequence typing (MLST). Of these, ST121 has been most commonly reported as a cause of SSSS. (4) Doudoulakakis et al had observed a sharp increase in SSSS settings since 2015, with 31 cases having been documented during the period 2014-2017. The molecular investigation of strains from that period demonstrated the emergence of a methicillin-susceptible, mupirocin- and fusidic acid-resistant Staphylococcus aureus clone belonging to the ST121 complex and carrying both epidermolysin (eta/etb) genes. (5) Additionally, while the SSSS exfoliation has been mainly attributed to the effects of the ETs, studies have also reported the Panton-Valentine leukocidin (PVL) genes associated with SSSS isolates. (4)
Clinical spectrum
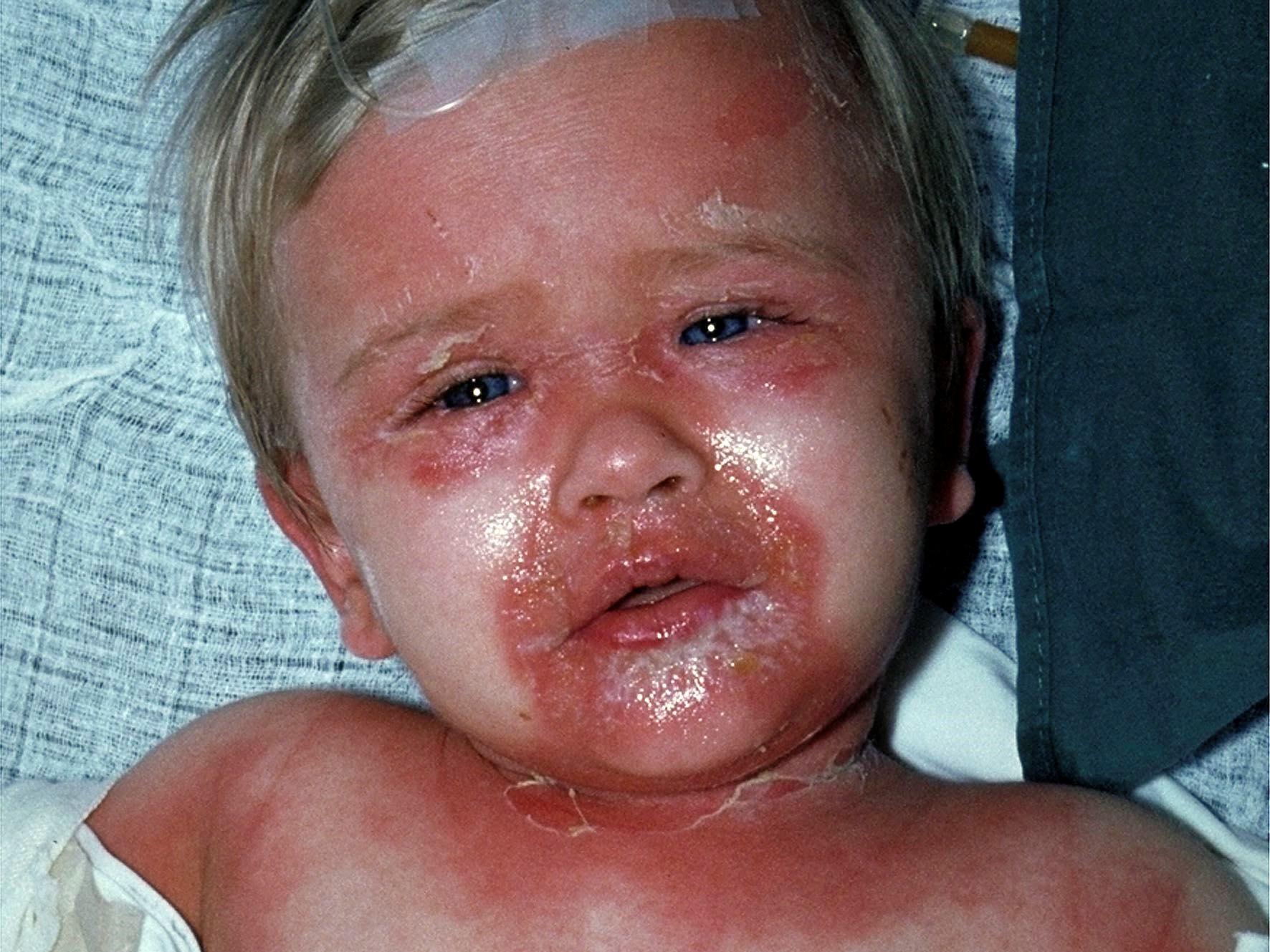
Mazori et al note that SSSS classically presents with acute‐onset, generalized, tender erythema with flexural accentuation, flaccid blisters, periorificial crusting and radial fissuring; the Nikolsky sign is positive. As previously discussed, although the blisters of SSSS are sterile, S. aureus may be cultured from sites of infection or colonization where the toxins originate prior to dissemination, notably the conjunctivae, nares, perioral skin, axillae, umbilicus and anus. Patients are characteristically febrile, irritable, lethargic and anorexic. Although this classic severe phenotype is widely recognized in children, SSSS is spectrum of disease with mild and moderate variants. The authors presented four variant cases in infants. Physical examination demonstrated varying degrees of nontender erythema and superficial desquamation with periorificial, flexural and/or acral accentuation. All patients had a negative Nikolsky sign. One patient was febrile; vital signs were otherwise normal in all patients. One patient had leukocytosis. Although the reason for the SSSS phenotypic variability is unknown, it has been hypothesized that toxin load may play a role, with milder phenotypes having increased renal toxin clearance and/or higher levels of neutralizing antibodies. The authors emphasize that appreciating the variability of SSSS is crucial as the condition is potentially fatal; however, with medical management, the mortality rate in children is as low as 0.33%. (6)
SSSS in adults is a different story. Patients at risk include those who are immunosuppressed (notably HIV/AIDS), have severe renal impairment or failure, or in those with malignancies. The mortality rate is much higher in adults than children, having been reported as high as 59%. (7)
Therapeutic approach
Wang et al retrospectively studied 51 patients with SSSS; sensitivity data were available for 28 patients. Resistance to clindamycin was observed in 17 isolates (63%), erythromycin in 18 isolates (69%), and trimethoprim/sulfamethoxazole in 2 isolates (7%). No isolates were resistant to oxacillin or vancomycin. The authors concluded SSSS-associated isolates were more likely to be clindamycin-resistant and less likely to be methicillin-resistant compared to overall staphylococcal infections. They favor cephalosporins and penicillinase-resistant penicillins (eg, oxacillin) for empiric management of SSSS, with consideration of adding MRSA coverage in communities with high MRSA prevalence or failure to improve following several days of treatment. Although clindamycin has been touted to reduce toxin production, the authors found no benefit to adding clindamycin to the regimen. (8)
Supportive care for management of dehydration, thermoregulation, and nutritional support is crucial. Emollients and non-adherent dressing should be applied to the skin and denuded areas to promote healing and reduce heat loss. (7)
In conclusion, even though it has been more than 140 years since it was first described, SSSS is a dynamic disorder with a spectrum of clinical presentations and an altered microbiology that demands our attention.
Point to remember: SSSS may present on a clinical spectrum and has been increasing in incidence. Although vastly more common in infants, adults with immunosuppression and renal impairment are at risk, with a much greater mortality. Changes in pathogenesis may be related to mupirocin resistance.
Our expert's viewpoint
Bernard Cohen, MD
Professor of Dermatology and Pediatrics
Johns Hopkins University School of Medicine
There is clear evidence that the incidence of SSSS has increased dramatically in the United States over the last 2 decades. As a result, it is important that we train our medical colleagues, particularly pediatricians, to recognize the clinical findings and to distinguish SSSS from toxic epidermal necrolysis/Stevens-Johnson syndrome. They need to know that the toxins target desmoglein 1, which is present high in the epidermis, but not in mucous membranes.
Interestingly, in our series at the Johns Hopkins Children's Center none of the affected children had an identifiable primary bacterial infection; positive cultures were obtained from sites of colonization including the nares, conjunctivae, umbilicus, and perianal area. There was some variability in the clinical presentations, but most demonstrated classical findings including diffuse erythema, erosions around sites of minor trauma (particularly intertriginous and periorificial areas), and a positive Nikolsky sign. In our series we excluded 2 children who developed SSSS during prolonged hospitalizations for complications of prematurity. None of our patients were immunodeficient, on immunosuppressive medications, or had decreased renal function.
Moreover, the length of hospitalization in our patients averaged 2 days, regardless of the antibiotic administered and whether or not the organism was sensitive or resistant to the antibiotic. Many clinicians have pushed for use of clinidamycin because it suppresses toxin production, but even in those patients who were given clindamycin montherapy, there was no change in length of hospitalization. Additionally, resistance of the causative organisms to clindamycin is approaching or exceeding 50%.
It makes me wonder if an antibiotic is helpful when a primary infection is not identified in otherwise healthy immunocompetent patients. I understand that the standard of care is administration of systemic antibiotics, and in light of our finding that 100% were MSSA, we recommend a cephalosporin and penicillinase-resistant penicillins with consideration of adding MRSA coverage in communities with a high incidence of MRSA, until culture and sensitivities are available.
Management of pain, fluids and electrolytes, and good skin care is critical. We discouraged narcotics since pain usually decreased dramatically within 36-48 hours with aggressive use of topical emollients — non-stick dressings and debridement was a no-no! Frequent application of emollients to the crusty vermillion border was critical to maintain oral intake in all patients, including bottle and breast feeding in young infants.
In short, SSSS is not rare, hospitalization in otherwise healthy children is usually short with proper management, and primary infections in healthy children is uncommon. Therapeutically, use of antibiotics may not change the generally favorable outcome if good skin care is provided; however, in children and adults with immunosuppression and/or renal disease the risk of morbidity and mortality is significant, and these patients need to be evaluated and treated aggressively.
Mockenhaupt M, Idzko M, Grosber M, Schöpf E, Norgauer J. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol 2005; 124: 700-703.
Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol 2018; 178: 704-708.
Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US adults.
Hultén KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing number of Staphylococcal scalded skin syndrome caused by ST121 in Houston, Texas. Pediatr Infect Dis J 2020; 39: 30-34.
Doudoulakakis A, Spilopoulou I, Syridou G, Giormezis N, et al. Emergence of staphylococcal scalded skin syndrome associated with a new toxinogenic, methicillin-susceptible Staphylococcus aureus clone. J Med Micobiol 2019; 68: 48-51.
Mazori DR, Leonard A, Alexander JB, Glick SA. The spectrum of staphylococcal scalded skin syndrome: A case series in children. Br J Dermatol 2020; 45: 333-336.
Ross A, Shoff HW. Staphylococcal scalded skin syndrome. StatPearls [Internet], Treasure Island (FL). StatPearls Publishing; 2020 Jan.
Wang Z, Feig JL, Mannschreck DB, Cohen BA. Antibiotic sensitivity and clinical outcomes in staphylococcal scalded skin syndrome. Pediatr Dermatol 2020; 37: 222-223.
Skin Care Physicians of Costa Rica
Clinica Victoria en San Pedro: 4000-1054
Momentum Escazu: 2101-9574
Please excuse the shortness of this message, as it has been sent from
a mobile device.
posted by dermatica at
April 28, 2021
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home