LIKE THE TUMOR ITSELF, KNOWLEDGE ABOUT DFSP IS EXPANDING WIDER AND DEEPER

By Warren Heymann, MD
June 16, 2021
Vol. 3, No. 24
DFSP is a low-to-intermediate grade sarcoma with a reported incidence of 4.1 per million person-years. Age at diagnosis is usually between 20 and 59 years, although it may be seen at any age, even congenitally. It is usually distributed on the trunk, more than the extremities or head and neck. DFSP is slightly more common in men and among Black people. It usually appears as a firm flesh-colored cutaneous and subcutaneous nodule(s) with slow growth. DFSPs are locally aggressive but metastasize in less than 5% of cases. The 10-year relative survival is > 99%; those with a worse prognosis tend to be older, male, Black, and have lesions on the extremities and head. (1,2)
Histologically, DFSP is derived from dermal fibroblasts that infiltrate adipose tissue; it can also develop directly from subcutaneous tissue. DFSP is a spindle cell tumor, with little atypia and mitotic activity, arranged in a storiform pattern, where cells often infiltrate in a honeycomb pattern. Immunohistochemically, spindle cells typically demonstrate strong expression of CD34 but are negative for other immunohistochemical stains used for other spindle cell neoplasms, including alpha-smooth muscle actin, factor XIIIa, S-100 and melan-A. CD34 expression may be reduced or lost in up to 45% of the fibrosarcomatous (FS) DFSP. CD34 expression is not pathognomonic for DFSP as it may be observed in spindle cell lipomas, fibromas, fibromyxomas and Kaposi sarcoma. DFSPs have multiple histological variants including myxoid, pigmented [Bednar tumor], giant cell giant cell fibroblastoma (GCF), granular cell, sclerotic, and FS-DFSP. These variants do not correlate with clinical manifestations or outcomes, except for the FS-DFSP, which has an increased risk of local recurrence and metastatic potential. (3) Regarding GCF, this variant is usually observed in children, although it may be observed at any age. Recent molecular analysis supports the concept that GCF is part of the DFSP spectrum (4)
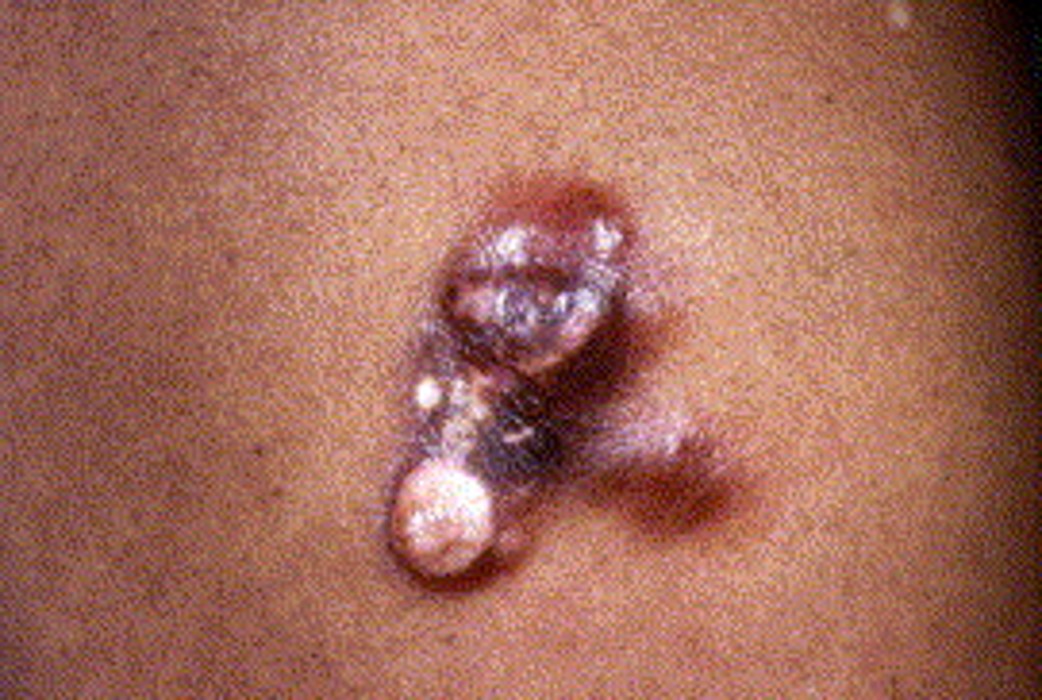
Differentiating a cellular dermatofibroma (CDF) from DFSP without immunoperoxidase stains is challenging. Schecter et al observed that all 20 cases of CDF demonstrated fat necrosis with a lymphocytic infiltrate in all 20 cases of CDF. None of 20 cases of DFSP had fat necrosis with a lymphocytic infiltrate, although 4 of 20 had fat necrosis alone. (5)
The pathogenesis of DFSP remains to be defined. Cases have been associated with sites of prior trauma, including burns, scars, tattoos, radiation, and vaccinations. (1) I have witnessed a case developing within a smallpox vaccination scar. (6) The role of immune status must always be considered — of interest is a case of rapid recurrence of a DFSP in a 75-year-old man within 4 months of adalimumab administration for his ankylosing spondylitis. (7)
A key to understanding the pathophysiology of DFSP is based on the finding that > 90% of tumors display a chromosomal translocation [t(17;22)(q22;q13)] with a resultant COL1A1-PDGFB fusion protein, which behaves as an autocrine growth factor; others cases may show different gene modifications. (1) Such an example is the case of a 42-year-old man presenting with a DFSP on the left cheek with foci of myxoid-fibrosarcomatous transformation. A conventional chromosomal analysis revealed a complex karyotype without a supernumerary ring chromosome or a linear translocation t(17;22). Comparative genome hybridization (CGH) and fluorescence in-situ hybridization (FISH) revealed the fusion of COL1A1 and PDGFB probes were inserted in chromosome 15. (8) According to Hao et al: "Interaction of PDGFB and PDGF receptor B is involved in multiple signaling pathways including Ras mitogen-activated protein kinases (RAS-MARK) and phosphatidylinositol 3-kinase-akt-rapamycin (mTOR) (PI3K-AKT-mTOR). Correspondingly, increased expression of the phosphorylated Akt-mTOR pathway proteins including Akt, mTOR, 4EBP1, and S6RP and phosphor-PDGFR A/B have been demonstrated in about half of DFSP tissues by immunoperoxidase studies, suggesting that Akt-mTOR pathways are involved in the tumorigenesis of DFSP."
While the main therapeutic approach to DFSP is surgical (Mohs micrographic surgery or wide local excision), imatinib is FDA approved for adults with unresectable, recurrent, or metastatic DFSP. (1) Imatinib is valuable in those cases with the characteristic translocation; in cases of imatinib resistance, using multikinase inhibitors such as sunitinib may be worthwhile. (3) In a study of 4 DFSPs with 92 dermatofibromas as controls, Park et al found that expression of Akt/mTOR, STAT3, ERK, and PD-L1 ranged from none or low in the primary skin lesions to high in the corresponding metastatic sites. Akt/mTOR and ERK were expressed more frequently in DFSP than in dermatofibromas, suggesting an association with the development and/or progression of DFSP. (9) Inhibition of these pathways may open new therapeutic horizons. There is a report of FS-DFSP responding to the mTOR inhibitor everolimus. (10) I am not aware of topical rapamycin being applied to DFSP, but it would make sense to try — even if it had only a minor effect on tumor shrinkage (without "skip" areas), that alone could help the surgeons.
Point to Remember: DFSPs are relatively rare lesions with the potential to be locally aggressive although metastatic disease is rare. Although optimal treatment is surgical, novel molecular insights offer new therapeutic possibilities.
Our expert's viewpoint
Kiran Motaparthi, MD
One of the common challenges encountered by dermatopathologists is differentiation of DF (particularly CDF) from DFSP based on partial or superficial sampling. Overlapping histopathologic and immunohistochemical features may be observed at the periphery of CDF and DFSP. (11) Peripheral collagen trapping is common in both DF and DFSP, as is a grenz zone with overlying epidermal hyperplasia. (5) The periphery of CDF is commonly reactive for CD34, due to CD34-positive dermal fibroblasts which are intermixed with the neoplastic cells. (12) Furthermore, in routine practice, factor XIIIa expression is commonly weak, focal, or negative in DF including CDF. CD163, an immunohistochemical marker that is highly specific for monocytes and macrophages, is expressed in 89% of DF and 100% of CDF but only 17% of DFSP. (13) As noted in Dr. Heymann's commentary, molecular techniques can provide diagnostic support when necessary. However, cost can limit access to this testing. In the recent study by Schecter et al., fat necrosis with chronic inflammation was specific for DF, but this relies on evaluation of punch or excisional specimens with extension to the subcutis. (5) Agarwal et al. found a significant difference when comparing Ki-67 in DF (mean proliferation index 39.2/mm2) to the superficial portion of standard DFSP (mean proliferation index 12.6/mm2). By Phosphohistone-H3 (PHH3), all cases of DF showed ≥ 1mitotic figure/mm2, while most superficial samples of DFSP demonstrated none. (11)
Adequate sampling to include the center of the tumor and the subcutis permits a cytologic and architectural assessment that is efficient and accurate when combined with experience. When only a superficial biopsy is provided, monotonous cytomorphology, cellularity, CD163 and/or CD34, and assessment of proliferation index or mitotic count can aid in the distinction of DF from DFSP.
De Antoni E, Brambullo T, Pescarini E, Salmaso R, et al. Dermatofibrosarcoma protuberans on tattooed skin: A case report. Adv Skin Wound Care 2020: 33: 104-108.
Brooks JB, Ramsey ML. Cancer, dermatofibrosarcoma protuberans. StatPearls[ [Internet], Treasure Island [FL]: StatPearls Publishing, Jan 2020.
Hao X, Billings SD, Wu F, Stulz TW, et al. Dermatofibrosarcoma protuberans: Update on the diagnosis and treatment. J Clin Med 2020; 9: E1752
Braswell DS, Ayoubi N, Motaparthi K, Walker A. Dermatofibrosarcoma protuberans with features of giant cell fibroblastoma in an adult. J Cutan Pathol 2020; 47: 317-320.
Schecter SA, Bresler SC, Patel RM. Fat necrosis with an associated lymphocytic infiltrate represents a histopathologic clue that distinguishes cellular dermatofibroma from dermatofibrosarcoma protuberans. J Cutan Pathol 2020 May 15 [Online ahead of print].
Green JJ, Heymann WR. Dermatofibrosarcoma protuberans occurring in a smallpox vaccination scar. J Am Acad Dermatol 2003; 48: S54-S55.
Hayama K, Fujita H, Fujikama M, Takahashi S, et al. Rapid recurrence of dermatofibrosarcoma protuberans after initiation of adalimumab therapy in a patient with ankylosing spondylitis. J Dermatol 2020; 47: e244-246.
Daoud A, Cunningham CR, Kozel JA, Slutsky JB, et al. A novel aberration of COL1A1-PDGFB fusion as an insertion in chromosome 15 in one case of dermatofibrosarcoma protuberans involving a rare location. J Cutan Pathol 2020; Jun 9 [Online ahead of print]
Park S, Cho S, Kim M, Park JU. Dermatofibrosarcoma protuberans: A retrospective study of clinicopathologic features and related Akt/mTOR, STAT3, ERK, cyclin D1 and PD-L1 expression. J Am Acad Dermatol 2018; 79: 843-852.
Stacchiotti S, Pedeutour F, Negri T, Conca E, et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: Clinical history, biological profile and sensitivity to imatinib. Int J Cancer 2011; 129: 1761 -1722.
Agarwal A, Gopinath A, Tetzlaff MT, Prieto VG. Phosphohistone-H3 and Ki67: Useful markers in differentiating dermatofibroma from dermatofibrosarcoma protuberans and atypical fibrohistiocytic lesions. Am J Dermatopathol. 2017; 39: 504-507.
Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012; 39: 747-752.
Sachdev R, Sundram U. Expression of CD163 in dermatofibroma, cellular fibrous histiocytoma, and dermatofibrosarcoma protuberans: comparison with CD68, CD34, and Factor XIIIa. J Cutan Pathol. 2006; 33: 353-360.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
Skin Care Physicians of Costa Rica
Clinica Victoria en San Pedro: 4000-1054
Momentum Escazu: 2101-9574
Please excuse the shortness of this message, as it has been sent from
a mobile device.
posted by dermatica at
June 16, 2021
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home