Vexas Syndrome and Mielodisplasia Cutis
THE NEXUS OF VEXAS SYNDROME

By Warren R. Heymann, MD, FAAD
December 1, 2021
Vol. 3, No. 47

To appreciate the clinical nuances of the VEXAS syndrome, it is imperative to understand the concept of ubiquitination. As previously discussed in the DermWorld Insights and Inquiries commentary "Ubiquitous Ubiquitination" ubiquitination regulates the function and signaling of a profusion of proteins in various cellular pathways. (1) Ubiquitination serves as a degradation mechanism of proteins as part of the ubiquitin-proteasome pathway but is also involved in additional cellular processes such as activation of the NFκB inflammatory response and DNA damage repair. Ubiquitin is a highly conserved 76-amino acid protein expressed in all cell types that can be polymerized into various linkages. Ubiquitination is a conserved multistep process that begins with the activation of ubiquitin with ATP by the E1 ubiquitin-activating enzyme. Ultimately, deubiquitinating enzymes remove ubiquitin from target substrates and recycle ubiquitin into the cytosolic pool. (2)
It has been long known that certain disorders, such as Sweet syndrome (SS) and relapsing polychondritis (RP), are associated with lymphoproliferative malignancies.
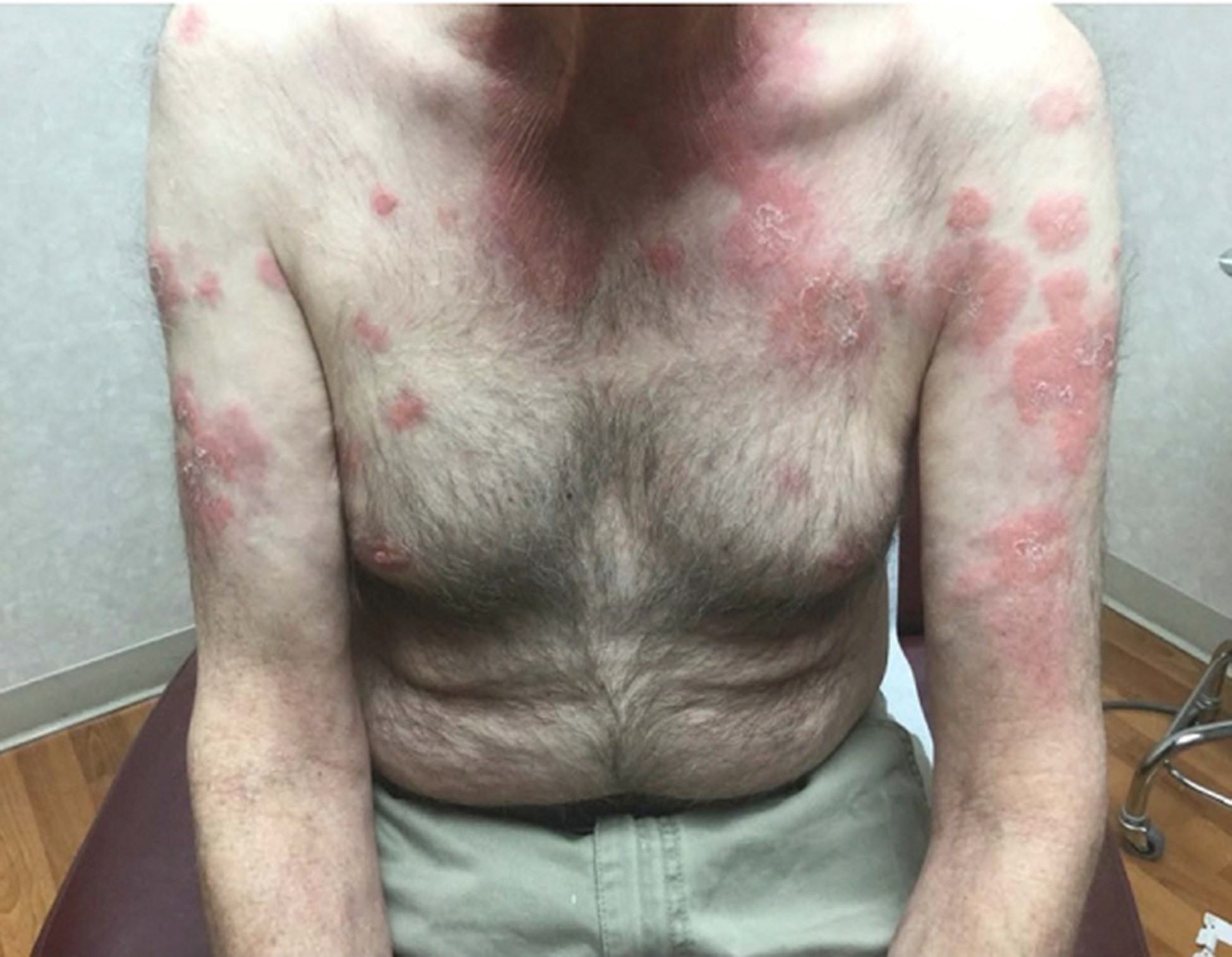
Beck et al used a genotype-driven approach to identify a disorder connecting seemingly unrelated adult-onset inflammatory syndromes, naming disorder the VEXAS. The authors identified 25 men with somatic mutations affecting methionine-41 (p.Met41) in UBA1, the major E1 enzyme that initiates ubiquitylation. (The gene UBA1 lies on the X chromosome.) These patients manifest an often fatal, treatment-refractory inflammatory syndrome that develops in late adulthood, with fevers, cytopenias, characteristic vacuoles in myeloid and erythroid precursor cells, dysplastic bone marrow, neutrophilic cutaneous and pulmonary inflammation, chondritis, and vasculitis. Most of these 25 patients met clinical criteria for an inflammatory syndrome (relapsing polychondritis, Sweet syndrome, polyarteritis nodosa, or giant-cell arteritis) or a hematologic condition (myelodysplastic syndrome or multiple myeloma) or both. Mutations were found in more than half the hematopoietic stem cells, including peripheral-blood myeloid cells but not lymphocytes or fibroblasts. Mutant peripheral-blood cells showed decreased ubiquitylation and activated innate immune pathways responsible for the clinical phenotype. (3)
Koster et al identified 9 men with VEXAS syndrome by finding somatic mutations in the UBA1 gene; the most frequent variant was p.Met41Thr. The median age was 74 years, and patients had a median duration of symptoms for 4 years prior to the diagnosis. Refractory constitutional symptoms (88%), ear and nose chondritis (55%), and inflammatory arthritis (55%) were common clinical features. Vasculitis was noted in 44%. All patients had significantly elevated inflammatory markers and macrocytic anemia. Thrombocytopenia was present in 66%. Eight patients had bone marrow biopsies performed. All bone marrows were hypercellular, and there was vacuolization of the erythroid (100%) or myeloid precursors (75%). Glucocorticoids improved symptoms (prednisone doses ≥20 mg per day), however other immunosuppressive agents did not provide consistent long-term control of the syndrome. The authors confirmed that VEXAS syndrome is a clinically heterogeneous, treatment-refractory inflammatory condition caused by somatic mutation of the UBA1 gene. Patients often present with overlapping rheumatologic manifestations and persistent hematologic abnormalities. (4) Sharma et al reported a case of VEXAS syndrome in a 70-year-old man with systemic lupus erythematosus. (5)
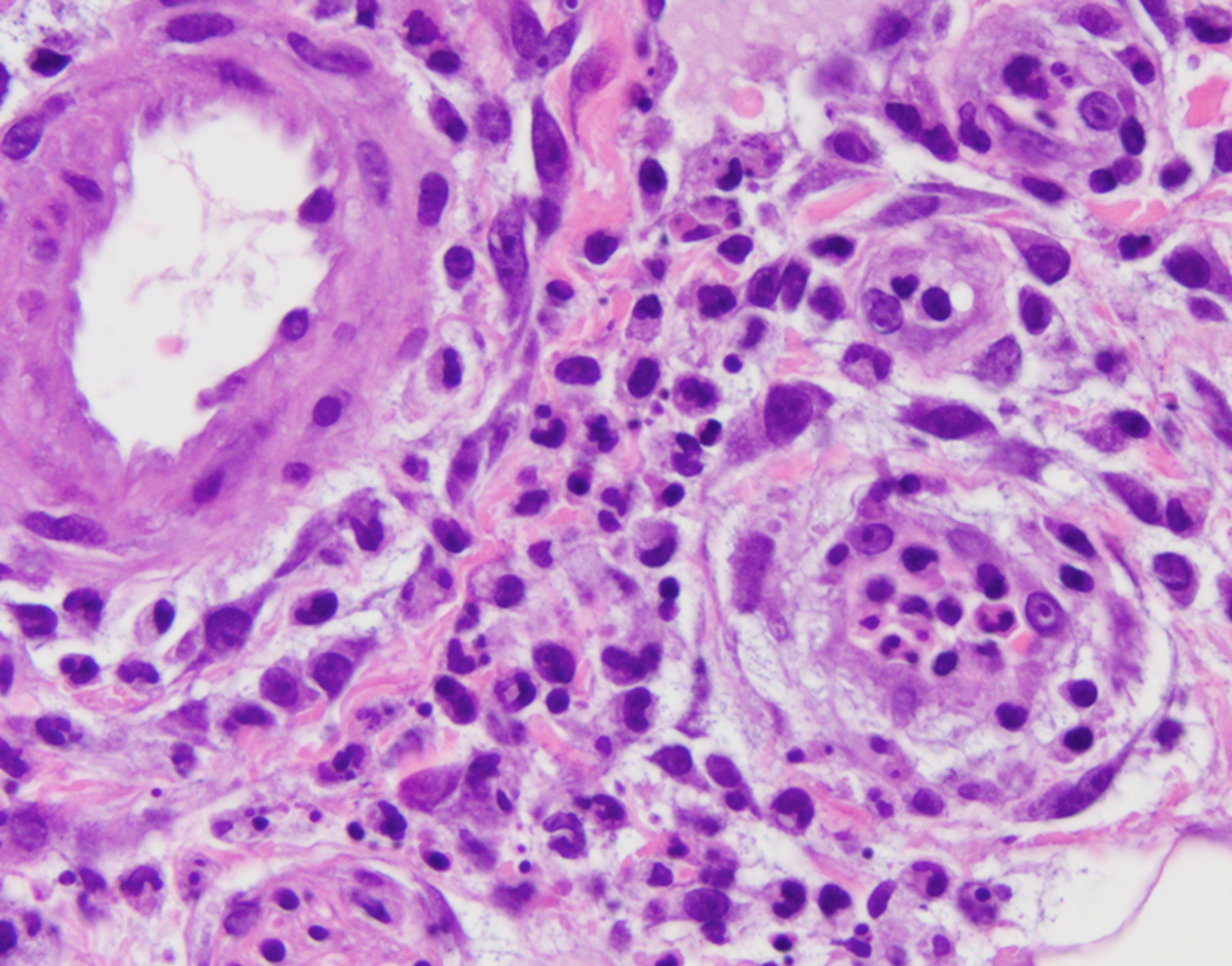
Zakine et al focused on the dermatological manifestations of VEXAS syndrome, identifying 8 men whose median age at symptom onset was 65.5 years. All patients had neutrophilic dermatosis skin lesions, including tender red or violaceous papules, sometimes edematous, inflammatory edematous papules on the neck and trunk (sometimes umbilicated), and firm erythematous purpuric or pigmented infiltrated plaques and nodules. Three patients had livedo racemosa. Three of 8 displayed fever and arthralgia, while 3 other patients had associated RP. Histologically, the infiltrates consisted of mature neutrophils with leukocytoclasia, which were admixed with myeloperoxidase-positive CD163-positive myeloid cells with indented nuclei and lymphoid cells in all cases. A sequencing analysis of paired bone marrow samples and skin lesion biopsies identified the same loss-of-function UBA1 variation in both samples for all patients, suggesting that the dermal infiltrates seen in VEXAS skin lesions are derived from the pathological myeloid clone. (6)
VEXAS syndrome may prove fatal in up to 40% of cases due to progressive anemia, respiratory failure, or complications of therapy. (3) Systemic steroids are the only effective therapy to date. Potential treatment modalities may include bone marrow transplantation or gene therapy. (7)
It will be fascinating to determine how many inflammatory disorders associated with myelodysplastic disease are due to UBA1 mutations. Ferrada et al performed exome and targeted sequencing of UBA1in a prospective observational cohort of patients with RP. Seven of 92 patients with RP (7.6%) had UBA1 mutations. The mortality was significantly greater in those designated as VEXAS-RP than in RP alone (23% versus 4%; P = 0.029). (8) I anticipate that similar studies will be performed with other disorders such as Sweet syndrome.
The famous postcard line "The sun has riz, the sun has set, and here we is in Texas yet" refers to the enormity of the state. Dermatologists are now entering the vast state of VEXAS and need to learn its geography.
Point to Remember: Think of VEXAS syndrome when seeing elderly men with neutrophilic dermatoses in combination with myelodysplasia and relapsing polychondritis. It is a prime example of how exome analysis can explain the co-occurrence of previously disparate disorders.
Our expert's viewpoint
Jean-David Bouaziz and Marie-Dominique Vignon-Pennamen, Saint Louis Hospital and Paris University, Paris, France
Neutrophilic dermatosis associated with VEXAS syndrome: When myeloid malignancy links with clonal autoinflammation
Sweet's syndrome (SS) was historically described as an acute disease with skin lesions, fever, and arthralgia. (9) Apart from this classical form, SS-like lesions occurring in elderly had sometimes a more chronic course and were strongly associated with myelodysplastic syndrome (MDS). (10) In this MDS-associated SS-like cutaneous disease, the bone marrow clonal origin of myeloid cells infiltrating the skin was clearly demonstrated by our group as evidenced by cytological (10), cytogenetic (same cytogenetic abnormalities in the skin and in the bone marrow) (11), and genomic studies (same oncogenic mutation in the skin and in the blood marrow). (12) To describe SS-like cutaneous disease in the setting of MDS, that does not result from germinal inborn errors in inflammasome pathways such as described in monogenic and polygenic disorders (for example pyoderma gangrenosum in Crohn's disease), the term Myelodysplasia Cutis was coined by us because of the bone marrow clonal origin of the immature myeloid cells in the skin. (13) In VEXAS syndrome, myeloid cells that infiltrate the skin have not only a clonal bone marrow origin (6) but also an activation of their inflammatory loops involving the ubiquitin pathway. Two questions remain: 1) it remains to be proved if the clonal infiltration of UBA1 myeloid cells in the skin of VEXAS syndrome could also be seen in the cartilage, the lung, and other inflamed organs; 2) in a recent series of patients with MDS associated inflammatory disorders the frequency of UBA1 mutation was 5% (4/85 patients) (14), suggesting that other oncogenic drivers may induce SS like Myelodysplasia Cutis skin lesions.
https://www.aad.org/dw/dw-insights-and-inquiries/dermatopathology/ubiquitous-ubiquitination
Gallo LH, Ko J, Donoghue DJ. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle. 2017 Apr 3;16(7):634-648. doi: 10.1080/15384101.2017.1288326. Epub 2017 Feb 6. PMID: 28166483; PMCID: PMC5397262.
Beck DB, Ferrada MA, Sikora KA, Ombrello AK, Collins JC, Pei W, Balanda N, Ross DL, Ospina Cardona D, Wu Z, Patel B, Manthiram K, Groarke EM, Gutierrez-Rodrigues F, Hoffmann P, Rosenzweig S, Nakabo S, Dillon LW, Hourigan CS, Tsai WL, Gupta S, Carmona-Rivera C, Asmar AJ, Xu L, Oda H, Goodspeed W, Barron KS, Nehrebecky M, Jones A, Laird RS, Deuitch N, Rowczenio D, Rominger E, Wells KV, Lee CR, Wang W, Trick M, Mullikin J, Wigerblad G, Brooks S, Dell'Orso S, Deng Z, Chae JJ, Dulau-Florea A, Malicdan MCV, Novacic D, Colbert RA, Kaplan MJ, Gadina M, Savic S, Lachmann HJ, Abu-Asab M, Solomon BD, Retterer K, Gahl WA, Burgess SM, Aksentijevich I, Young NS, Calvo KR, Werner A, Kastner DL, Grayson PC. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N Engl J Med. 2020 Dec 31;383(27):2628-2638. doi: 10.1056/NEJMoa2026834. Epub 2020 Oct 27. PMID: 33108101; PMCID: PMC7847551.
Koster MJ, Kourelis T, Reichard KK, Kermani TA, Beck DB, Cardona DO, Samec MJ, Mangaonkar AA, Begna KH, Hook CC, Oliveira JL, Nasr SH, Tiong BK, Patnaik MM, Burke MM, Michet CJ Jr, Warrington KJ. Clinical Heterogeneity of the VEXAS Syndrome: A Case Series. Mayo Clin Proc. 2021 Sep 3:S0025-6196(21)00481-X. doi: 10.1016/j.mayocp.2021.06.006. Epub ahead of print. PMID: 34489099.
Sharma A, Naidu G, Deo P, Beck DB. VEXAS syndrome with systemic lupus erythematosus- expanding the spectrum of associated conditions. Arthritis Rheumatol. 2021 Aug 30. doi: 10.1002/art.41957. Epub ahead of print. PMID: 34463053.
Zakine E, Schell B, Battistella M, Vignon-Pennamen MD, Chasset F, Mahévas T, Cordoliani F, Adès L, Sébert M, Delaleu J, Jachiet M, Lepelletier C, Lemaire P, Chauvel C, Dhouaieb B, Kim R, Cassius C, Georgin-Lavialle S, Mekinian A, Bagot M, Braun T, Rousset L, Begon E, de Masson A, Fenaux P, Clappier E, Bouaziz JD. UBA1 Variations in Neutrophilic Dermatosis Skin Lesions of Patients With VEXAS Syndrome. JAMA Dermatol. 2021 Sep 8. doi: 10.1001/jamadermatol.2021.3344. Epub ahead of print. PMID: 34495287.
Alhomida F, Beck DB, George TI, Shaffer A, Lebiedz-Odrobina D, Kovacsovics T, Madigan LM. Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome-clinical presentation of a newly described somatic, autoinflammatory syndrome. JAAD Case Rep. 2021 Jun 26;14:111-113. doi: 10.1016/j.jdcr.2021.06.010. PMID: 34337120; PMCID: PMC8313797.
Ferrada MA, Sikora KA, Luo Y, Wells KV, Patel B, Groarke EM, Ospina Cardona D, Rominger E, Hoffmann P, Le MT, Deng Z, Quinn KA, Rose E, Tsai WL, Wigerblad G, Goodspeed W, Jones A, Wilson L, Schnappauf O, Laird RS, Kim J, Allen C, Sirajuddin A, Chen M, Gadina M, Calvo KR, Kaplan MJ, Colbert RA, Aksentijevich I, Young NS, Savic S, Kastner DL, Ombrello AK, Beck DB, Grayson PC. Somatic Mutations in UBA1 Define a Distinct Subset of Relapsing Polychondritis Patients With VEXAS. Arthritis Rheumatol. 2021 Mar 28. doi: 10.1002/art.41743. Epub ahead of print. PMID: 33779074.
Sweet RD. An Acute Febrile Neutrophilic Dermatosis. Br J Dermatol. 1964 Aug-Sep;76:349-56. doi: 10.1111/j.1365-2133.1964.tb14541.x. PMID: 14201182.
Vignon-Pennamen MD, Juillard C, Rybojad M, Wallach D, Daniel MT, Morel P, Verola O, Janin A. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia: a report of 9 cases. Arch Dermatol. 2006 Sep;142(9):1170-6. doi: 10.1001/archderm.142.9.1170. PMID: 16983004.
Sujobert P, Cuccuini W, Vignon-Pennamen D, Martin-Garcia N, Albertini AF, Uzunov M, Redjoul R, Dombret H, Raffoux E. Evidence of differentiation in myeloid malignancies associated neutrophilic dermatosis: a fluorescent in situ hybridization study of 14 patients. J Invest Dermatol. 2013 Apr;133(4):1111-4. doi: 10.1038/jid.2012.408. Epub 2012 Nov 29. PMID: 23190893.
Passet M, Lepelletier C, Vignon-Pennamen MD, Chasset F, Hirsch P, Battistella M, Duriez P, Sicre de Fontbrune F, Boissel N, Legrand O, Raffoux E, Bagot M, Adès L, Clappier E, Bouaziz JD. Next-Generation Sequencing in Myeloid Neoplasm-Associated Sweet's Syndrome Demonstrates Clonal Relation between Malignant Cells and Skin-Infiltrating Neutrophils. J Invest Dermatol. 2020 Sep;140(9):1873-1876.e5. doi: 10.1016/j.jid.2019.12.040. Epub 2020 Feb 17. PMID: 32081610.
Delaleu J, Battistella M, Rathana K, Vignon-Pennamen MD, Laurent C, Ram-Wolff C, Fenaux P, Jachiet M, Zuelgaray E, Bagot M, Bouaziz JD, de Masson A. Identification of clonal skin myeloid cells by next-generation sequencing in myelodysplasia cutis. Br J Dermatol. 2021 Feb;184(2):367-369. doi: 10.1111/bjd.19547. Epub 2020 Dec 16. PMID: 32964412.
Zhao LP, Schell B, Sébert M, Kim R, Lemaire P, Boy M, Mathis S, Larcher L, Chauvel C, Dhouaieb MB, Bisio V, Preudhomme C, Marceau-Renaut A, Itzykson R, Mekinian A, Fain O, Toubert A, Fenaux P, Dulphy N, Clappier E, Adès L. Prevalence of UBA1 mutations in MDS/CMML patients with systemic inflammatory and auto-immune disease. Leukemia. 2021 Sep;35(9):2731-2733. doi: 10.1038/s41375-021-01353-8. Epub 2021 Aug 3. PMID: 34344988.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
Skin Care Physicians of Costa Rica
Clinica Victoria en San Pedro: 4000-1054
Momentum Escazu: 2101-9574
Please excuse the shortness of this message, as it has been sent from
a mobile device.
posted by dermatica at
December 01, 2021
![]()
![]()

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home